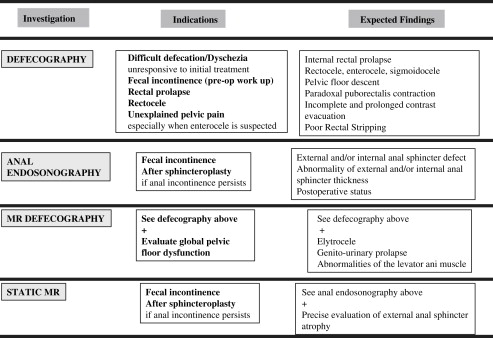
Several imaging modalities are available ranging from fluoroscopic techniques to ultrasonography and MRI for the evaluation of patients with pelvic floors disorders. High-resolution ultrasonography and MRI not only provide superior delineation of the pelvic floor anatomy but also reveal pathology and functional changes. This article focuses on standard imaging procedures including defecography, ultrasonography, and MRI and discusses its use in clinical practice by illustrating both normal and abnormal patterns.
Pelvic floor disorders refer to a group of clinical conditions that include pelvic organ prolapse, urinary and fecal incontinence, chronic constipation, and pelvic pain. Various imaging modalities are available, including ultrasound (US), MRI, and defecography. Over the last decade there have been considerable advances in several imaging techniques. High-resolution US and MRI not only provide superior depiction of the pelvic anatomy but also help to understand pathology and functional changes. MRI can also be used for a multicompartmental dynamic assessment of the pelvic floor (dynamic MRI defecography) and for the analysis of the sphincters (static MRI). Because MRI has enormous potential, it underscores the need for an integrated evaluation of pelvic floor disorders that involves the radiologist and a multidisciplinary team.
This article focuses on defecography, anal US, and dynamic MRI by discussing normal and abnormal patterns and clinical use. Particular emphasis has been placed on the gastroenterologist’s point of view.
Defecography
Defecography (evacuation proctography) is a dynamic fluoroscopic examination ( Box 1 ). It began in 1964 with Burhenne’s dynamic barium studies of the defecation process. Defecography examines the dynamic changes of the perineum and the evacuation of the anorectum. This test is indicated in patients with constipation to identify an outlet obstruction caused by either anatomic or functional disorder. The procedure requires rectal opacification. Additionally, small bowel opacification, or in women vaginal opacification, may be performed. If bladder dysfunction is suspected, a cystography can be performed along with defecography in a one-session examination (cystodefecography).
Defecography is contraindicated in young women during menstruation and pregnancy.
The exact procedure should be clearly explained to obtain full cooperation from the patient. A quiet radiology suite is important, and the patient should be hidden from the view of the technologist to provide privacy during the act of defecation.
When an enterocele is suspected, a barium meal is administered 1.5 hours before the examination. In women, vaginal opacification with barium is recommended before rectal opacification. The latter is performed with a mixture of 150 to 200 mL of barium and a starchy component.
First, a lateral view is taken in the upright position to localize the bony landmarks and to check the quality of the various opacifications (small bowel, vagina, and rectum). Next, the patient is seated on a special commode for filming the dynamic process of defecation. Dynamic images are performed at rest, during squeeze, and during straining.
The pubococcygeal line is drawn on the lateral view. Then, the distance between this line and the anorectal junction is measured. The exact measurement of the anorectal angle is somewhat controversial.
Normal Findings
Normal patterns have been described. At rest, the anorectal junction is located above the ischium. During squeezing, the anorectal junction is elevated and located less than 3.5 cm below the pubococcygeal line. During the defecation process, the puborectalis muscle opens widely without anal or rectal prolapse and the anorectal junction descends below the pubococcygeal line. A rectocele, less than 2.5 cm in size, is inconsequential provided it empties completely at the end of defecation.
Pathology and Clinical Use
Defecography can reveal several structural abnormalities. The most frequent abnormal findings are perineal descent at rest or during squeezing; rectocele (significant if >3.5 cm in depth and with residual barium at the end of defecation); rectal prolapse; paradoxical contraction of the puborectalis muscle (dyssynergia); enterocele; and sigmoidocele ( Figs. 1 and 2 ). Interobserver agreement was shown to be good for the diagnosis of rectocele and enterocele but inadequate for perineal descent. Functional findings are also important. For example, an incomplete and prolonged contrast evacuation seems more specific of dyssynergia than an inappropriate puborectal contraction.
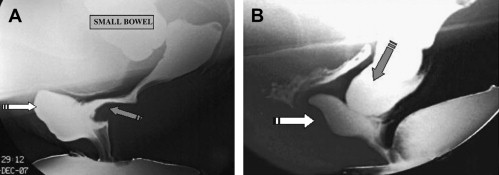
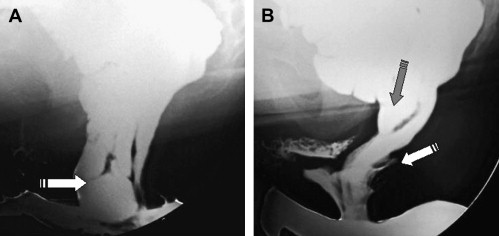
Defecography is indicated when some of the aforementioned abnormalities are clinically suspected, but not proved at clinical examination or in patients with an unexplained anorectal disorder. Defecography can be useful in cases of dyschezia (incomplete or difficult rectal emptying) when the patient did not respond to the first-line treatment ( Fig. 3 ). It can also be discussed in patients incontinent for solid stools before surgery if a pelvic static disorder (eg, a rectal prolapse) is suspected. Finally, it can be performed in patients with pelvic pain to rule out an enterocele. Rectocele and severe rectal prolapse could lead to surgery. This test is especially important when surgical treatment is being considered for a problem, such as rectal prolapse. The association of an enterocele can modify the surgical option. The relevance of defecography findings, however, remains controversial. In many instances, normal individuals show abnormal findings and hence the link between a clinical symptom and an abnormal defecography finding is difficult to establish.
Ultrasonography
With the advent of the newest transducer technology, US imaging is gaining a key role in the understanding of pelvic floor disorders. The most commonly used technique is endoanal US but endovaginal and transperineal US techniques are being developed and represent potential new uses. Additionally, the use of three-dimensional software may provide an accurate diagnosis of complex diseases.
Anal Endosonography
Anal endosonography is a technique specially adapted for the examination of anal sphincters ( Box 2 ). The main indication is the diagnosis of anal sphincter defect in the investigation of patients with fecal incontinence. Anal endosonography was first developed in the 1990s at Saint Mark’s Hospital, London, UK. It is also useful in the assessment of anal sepsis, anal cancer, and perineal pain.
A rectal enema is recommended a few hours before the examination. An US machine equipped with an anorectal transducer (eg, Bruel and Kjaer) is required. The rigid cylindric transducer offers 360-degree high-resolution image with a frequency ranging from 6 to 16 MHz.
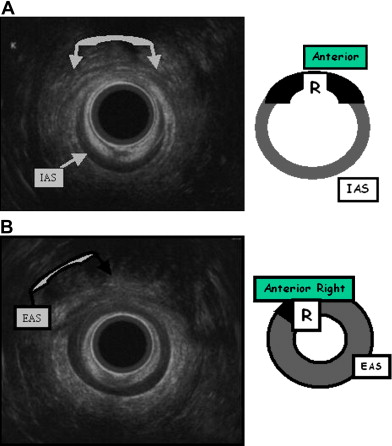
The patient is placed in the supine (or left lateral) position throughout the examination. The anal transducer is gently introduced and slowly withdrawn to obtain several images of the anal canal and surroundings: at the upper part of the anal canal the puborectalis muscle is identified, then both the external anal sphincter and internal anal sphincter are visible. Finally at the lower end, only the external anal sphincter is visible. The patient may be asked to contract voluntarily during the examination. Location and size of abnormalities are based on quadrants using a standard clock face, 12 o’clock being the anterior midline point. The external anal sphincter and internal anal sphincter thickness is also assessed.
Normal Findings
A good knowledge of the anatomy of anal sphincters and pelvic floor is required. From the surface outward, several layers can be identified ( Fig. 4 ). Mucosa and submucosa usually appear hyperechoic. The 2- to 3-mm thick internal anal sphincter (IAS) is in continuity with the circular muscular layer of the rectum. It is clearly delineated, homogeneously hypoechoic, and its thickness increases with age. The hyperechoic longitudinal muscle layer of rectum is difficult to distinguish from the hyperechoic external anal sphincter (EAS). At its upper part, the EAS is in continuity with the puborectalis muscle. It appears annular, thick, and symmetric in men and it is thinner and anteriorly opened in women. Its subcutaneous part is highly hyperechoic. Its mean thickness is 6 to 8 mm. Other elements can be identified: the “U-shaped” puborectalis muscle, the hypoechoic anococcygeal ligament, the ischioanal fatty spaces, the vagina, and the urethra.
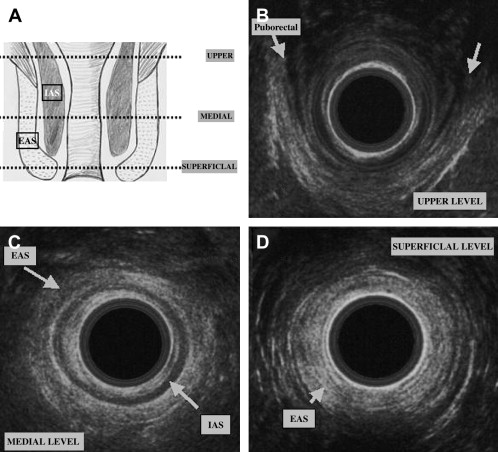
Pathology and Clinical Use
Anal endosonography is commonly performed to identify an EAS or IAS defect that may cause fecal incontinence. An IAS defect is diagnosed based on a segmental loss of circumference and retraction of the torn ends ( Fig. 5 ). An EAS defect is diagnosed based on a sharply delineated hypoechoic area that interrupts the normal echostructure (see Fig. 5 ). Both IAS and EAS defects can be present. The anterior part of the EAS in women needs to be analyzed carefully; it often appears thin and slightly heterogeneous because of its muscular insertion (tendinous arch of the pelvis). The anococcygeal ligament is responsible for a hypoechoic triangle, which should not be misdiagnosed as a pathologic defect.
Anal endosonography can detect sphincter defects with great accuracy. The sensitivity and specificity of anal endosonography for the diagnosis of anal sphincter defect is over 90%. Obstetric trauma remains the main cause of sphincter disruption and represents a major health problem. Anorectal trauma is a consequence of surgery for fistula in ano, hemorrhoids, or anal fissure. In these iatrogenic situations, the location of the defect correlates well with the surgical procedure.
Anal endosonography is clinically useful in patients with fecal incontinence when an anal sphincter disruption is suspected (see Fig. 3 ). Therapy can be directed based on US findings, such as the type (external or internal) and the size of the defect often expressed in degrees or percentage of the anal circumference. EAS defects that occupy less than 120 degrees of the anal circumference can be surgically corrected by overlapping sphincter repair. Anal endosonography can also be performed after a sphincteroplasty to assess the morphology of the EAS and to correlate surgery with the functional results. In patients with surgical repair of the EAS, the outcome was more favorable when there was evidence that the sphincter ends were overlapping. Anal endosonography can accurately demonstrate abnormal IAS thickness because the borders of this smooth muscle are sharply limited. In contrast, EAS atrophy is more difficult to identify by means of two-dimensional anal endosonography. Recently, three-dimensional anal endosonography has been described and seems promising.
Endovaginal and Transperineal Ultrasonography
Pelvic floor dysfunction in women may occur secondary to morphologic and anatomic changes of the urogenital organs and these can be identified by perineal and transvaginal US. US as part of the diagnostic work-up of stress urinary incontinence and urinary prolapse allows morphologic and dynamic assessment of the lower urinary tract. Anal sphincters can be analyzed by both approaches.
Transperineal US, also called “translabial” or “introital” US, allows assessment of the bladder neck and urethral motility using a 5- to 7-MHz transducer. For this test, the bladder should be full. The patient lies on her back and the probe is placed between the labia and gentle pressure is applied to the introitus and the external urethral orifice. The transducer axis corresponds to the body axis. The patient is asked to perform certain maneuvers (coughing and contraction of pelvic floor muscles) to evaluate the changes in position of the urethra and bladder.
Transvaginal US is a well-established procedure in gynecologic practice and can be useful. Here with the patient lying on her back, the transducer is gently applied to the dorsal vaginal wall. It provides very detailed information about the pelvic floor and in particular the area surrounding the urethral sphincter complex.
The aforementioned approaches are under clinical evaluation. There are no evidence-based data for their clinical use in patients with stress urinary incontinence or urinary prolapse.
Ultrasonography
With the advent of the newest transducer technology, US imaging is gaining a key role in the understanding of pelvic floor disorders. The most commonly used technique is endoanal US but endovaginal and transperineal US techniques are being developed and represent potential new uses. Additionally, the use of three-dimensional software may provide an accurate diagnosis of complex diseases.
Anal Endosonography
Anal endosonography is a technique specially adapted for the examination of anal sphincters ( Box 2 ). The main indication is the diagnosis of anal sphincter defect in the investigation of patients with fecal incontinence. Anal endosonography was first developed in the 1990s at Saint Mark’s Hospital, London, UK. It is also useful in the assessment of anal sepsis, anal cancer, and perineal pain.
A rectal enema is recommended a few hours before the examination. An US machine equipped with an anorectal transducer (eg, Bruel and Kjaer) is required. The rigid cylindric transducer offers 360-degree high-resolution image with a frequency ranging from 6 to 16 MHz.
The patient is placed in the supine (or left lateral) position throughout the examination. The anal transducer is gently introduced and slowly withdrawn to obtain several images of the anal canal and surroundings: at the upper part of the anal canal the puborectalis muscle is identified, then both the external anal sphincter and internal anal sphincter are visible. Finally at the lower end, only the external anal sphincter is visible. The patient may be asked to contract voluntarily during the examination. Location and size of abnormalities are based on quadrants using a standard clock face, 12 o’clock being the anterior midline point. The external anal sphincter and internal anal sphincter thickness is also assessed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







