Marc Goldstein, MD, DSc (Hon)
Since the previous edition, the indications for and techniques of surgery for male infertility have been significantly refined, resulting in substantially increased success in the management of male factor infertility. These advances include (1) increasing use of genetic and molecular biologic markers (see Chapters 20 and 21) to better select patients for surgical treatment; (2) improved techniques for microsurgical reconstruction for obstruction; (3) the use of varicocelectomy for enhancement of spermatogenesis in azoospermic or severely oligospermic men (Matthews et al, 1998; Kim et al, 1999); and (4) refined microsurgical techniques for sperm retrieval combined with in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) for men with nonobstructive azoospermia. Even men with nonobstructive azoospermia due to Klinefelter syndrome, once regarded as hopeless cases, can now father biologic offspring with assisted reproductive techniques (Tournaye et al, 1996; Palermo et al, 1998; Ramasamy et al, 2009).
Use of transrectal high-resolution ultrasound, as well as scrotal ultrasound with color flow Doppler, has substantially improved our diagnostic and therapeutic abilities. Transrectal ultrasound of the seminal vesicles provides not only diagnostic information but ultrasound-guided aspiration of the seminal vesicles that allows retrieval of sperm to be used for IVF with ICSI (Jarow, 1996). Power Doppler may allow identification of pockets of sperm production in the testis, which may help guide sperm retrieval in men with nonobstructive azoospermia (Har-Toov et al, 2004; Herwig et al, 2004; Tunc et al, 2005).
IVF with ICSI has expanded our ability to treat even the most severe forms of male-factor infertility such as unreconstructable reproductive tract obstruction and nonobstructive azoospermia. It is, however, a costly procedure and an intense process for the female partner, with associated risks of complications including ovarian hyperstimulation, multiple gestations, and complications of the procedures for oocyte retrieval. Furthermore, as ICSI bypasses all natural biologic barriers, it raises realistic concerns of passing genetic abnormalities to the offspring (Kim et al, 1998; Foresta et al, 2005). Thus proper selection of couples for advanced assisted reproductive technology, along with adequate genetic counseling, is mandatory.
On the other hand, recent analyses clearly indicate that specific treatments for male-factor infertility such as microsurgical reconstruction for obstructive azoospermia and varicocelectomy for impaired testis function, in properly selected patients, remain the safest and most cost-effective ways of managing infertile men (Kolettis and Thomas, 1997; Pavlovich and Schlegel, 1997; Lee et al, 2008). Specific treatment aimed at correcting or enhancing male infertility can upgrade a couple from intensive levels of assisted reproduction to simpler methods or even to naturally conceived pregnancies.
For men with unreconstructable obstruction, as well as men with nonobstructive azoospermia, surgical retrieval of sperm to achieve fertilization, pregnancy, and live birth with IVF and ICSI is a feasible management option. The development and recent refinement of the various techniques of surgical sperm retrieval, from testes, epididymides, or seminal vesicles with percutaneous or open surgical approaches, have expanded the armamentarium of urologists treating infertile men. In particular, the employment of the operating microscope to evaluate and identify individual seminiferous tubules more likely to contain sperm has significantly improved the success of testicular sperm extraction (Schlegel, 1999) while minimizing morbidity significantly (Tsujimura et al, 2002; Ramasamy et al, 2005).
The use of microsurgical techniques has also been extended to varicocelectomy. Varicoceles have long been known to be associated with male infertility and have now clearly been shown to result in progressive, duration-dependent testicular injury (Russell, 1957; Lipshultz and Corriere, 1977; Nagler et al, 1985; Gorelick and Goldstein, 1993; Sigman and Jarow, 1997). Furthermore, microsurgical varicocelectomy, previously reserved only for men with oligospermia, has now been applied to men with nonobstructive azoospermia, resulting in induction of spermatogenesis and successful return of sperm to the ejaculate in many cases (Matthews et al, 1998; Kim et al, 1999; Pasqualotto et al, 2003; Pasqualotto et al, 2006; Ishikawa et al, 2008). Although varicocelectomy has historically been reserved for the treatment of infertile men and varicocele-induced pain, there is an emerging concept of early repair of varicoceles to prevent both future infertility and Leydig cell dysfunction. Substantial evidence has accumulated suggesting that varicocele adversely affects Leydig cell function, resulting in lower serum testosterone levels when compared with age-matched controls without varicocele. Varicocelectomy can halt and even partially reverse this decline (Castro-Magana et al, 1989; Su et al, 1995; Cayan et al, 1999). In selected men, varicocelectomy may be an effective treatment for symptomatic, age-related androgen deficiency (Tanrikut et al, 2011) a condition increasingly referred to as andropause or testosterone deficiency syndrome (TDS). Thus with safer and more effective microsurgical techniques, early varicocelectomy has expanded the urologist’s role from that of salvaging remaining testicular function to that of preventing future infertility and TDS.
Surgical Anatomy
The scrotal contents are unique in their accessibility for physical examination, imaging modalities, and surgical intervention. The success of surgery for male infertility and scrotal disorders is predicated on selection of the correct operation and the most appropriate surgical approach. The details of the history and careful physical examination followed by confirmatory, judiciously selected laboratory and imaging procedures are presented in Chapter 21. When surgical intervention for diagnostic or therapeutic purposes is indicated, a thorough understanding of the anatomy (see Chapter 2) and physiology (see Chapter 20) of the male reproductive system is requisite for planning and carrying out a surgical procedure with the highest probability of success and lowest morbidity.
The key points of surgical anatomy follow.
Testicular Blood Supply
The main blood supply to the testis is from the testicular (internal spermatic) artery arising directly from the aorta (Table 22–1). A second blood supply comes from the artery of the vas deferens (deferential artery), which derives from the hypogastric (internal iliac) artery or the superior vesicle artery (also a branch of the hypogastric). The third blood supply, primarily to the tunica vaginalis but with branches going to the testes, comes from the cremasteric (external spermatic) artery, which derives from the inferior epigastric artery. The testicular artery is the main blood supply to the testes. Its diameter exceeds the diameter of the deferential (vasal) artery plus the cremasteric artery combined (Raman and Goldstein, 2004). Although vasal and cremasteric arteries can provide adequate blood supply to the testes in the event that the testicular artery is ligated, especially in children, atrophy and/or azoospermia has resulted from testicular artery ligation both in adults and children. Experience with the one-stage Fowler-Steven operation for orchiopexy, in which the testicular artery is intentionally ligated, indicates that 20% to 40% of such testes atrophy, although the rate of atrophy is lower in the staged procedure.
Table 22–1 Blood Supply to Testis, Epididymis, and Vas Deferens
| Testis |
| Epididymis |
| Vas Deferens |
Special attention should be paid to men who have undergone vasectomy because the vasal artery has likely been compromised. In these men, maintaining the integrity of the testicular artery in any future operations such as varicocelectomy is critical (Lee JS et al, 2007).
Epididymal Blood Supply
The epididymis has a rich blood supply (see Table 22–1). The superior and medial epididymal arteries derive from the testicular artery. The blood supply to the cauda (inferior pole) of the epididymis derives from the vasal (deferential) artery. The two main blood supplies to the epididymis, running superiorly and inferiorly, form an extensive interconnection such that if the vasal artery is ligated from previous vasectomy, the blood supply to the epididymis from the testicular artery is more than adequate. In addition, in preparation for vasoepididymostomy or vasovasostomy, the epididymis can be intentionally dissected off of the testis and mobilized to the caput (see Technique When Vasal Length Is Severely Compromised) with the inferior and medial epididymal arteries intentionally ligated without adverse consequence. As long as the superior epididymal artery remains intact, the blood supply to the epididymis will be adequate.
Blood Supply of the Vas Deferens
The vas deferens obtains its blood supply from two sources (see Table 22–1). The seminal vesical (abdominal) end of the vas derives its blood supply from the deferential (vasal) artery, a branch of the internal iliac (hypogastric) artery or of its branch, the superior vesicle artery. The testicular end of the vas receives additional blood supply from the inferior epididymal arterial interconnections, which extends onto the vas deferens. The two blood supplies to the vas deferens freely anastomose with each other. After vasectomy, if the vasal vessels are ligated, the testicular end of the vas receives all of its blood supply from branches of the testicular artery and epididymal artery, whereas the seminal vesical (abdominal) end of the vas receives all of its blood supply from the deferential artery. The vas deferens receives no blood supply from the surrounding cremaster muscle or from any blood vessels from the spermatic cord. Therefore if the vas deferens is sectioned or obstructed in two different locations, the intervening segment will fibrose due to lack of blood supply. Therefore two simultaneous vasovasostomies cannot be safely performed on the same vas if the vasal vessels have been interrupted in both locations.
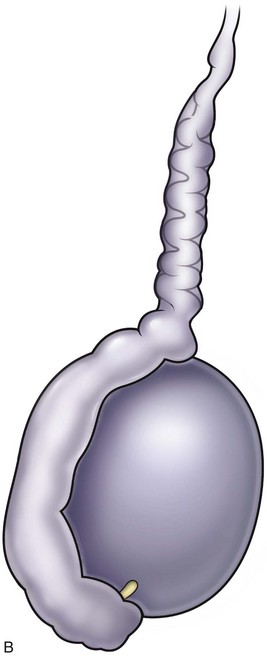
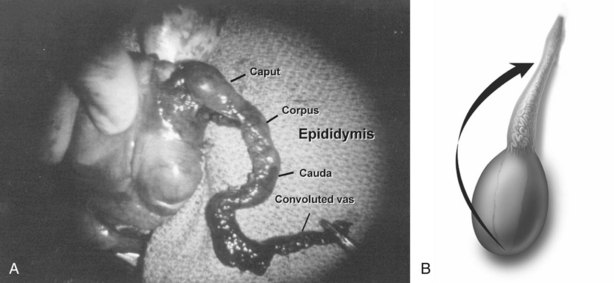
Anatomy of the Excurrent Ducts
Sperm and testicular fluid exit the testes through 7 to 11 tiny efferent ducts. These ducts become convoluted when they exit the testes and form the caput of the epididymis (see Chapter 20). At that level, they freely anastomose with each other. They all coalesce at the distal caput to form a single epididymal tubule from the caput-corpus junction all the way to the vas deferens. Therefore if the epididymis is accidentally injured or ligated distal to the caput, the entire system on that side will be completely obstructed. This is an important consideration when performing epididymal surgery or surgery near the epididymis. Hydrocelectomy is a common surgical procedure that can result in iatrogenic injury to the epididymis. In long-standing large hydroceles, the epididymis is often splayed out and difficult to identify. Generous margins from the epididymis should be allowed when performing hydrocelectomy (see Chapter 37). Orchiopexy for torsion can also result in inadvertent injury to the epididymis. A single stitch through an epididymal tubule in the corpus or cauda will result in complete obstruction of that side. Because there are multiple lobules at the levels of the caput, puncture of a single tubule for sperm aspiration can be safely performed at the most proximal region of the caput without significantly compromising the flow of sperm into the corpus. Multiple punctures of many tubules at the caput, or any puncture distal to the caput, however, can cause obstruction.
Ejaculatory Ducts
The left and right ejaculatory ducts enter the prostatic urethra at the level of the utricle. Obstruction of ejaculatory ducts can lead to azoospermia. Transurethral resection of the ejaculatory ducts (TURED) can relieve the obstruction. TURED should not be considered a benign procedure because it is occasionally associated with significant morbidity (see Transurethral Resection of the Ejaculatory Ducts later). Normally, the ejaculatory ducts contain a valvelike mechanism that prevents reflux of urine into the ejaculatory duct. After transurethral resection of the ejaculatory ducts, a significant percentage of men will develop reflux of urine up the excurrent ductal system (Vazquez-Levin et al, 1994), causing chemical and/or bacterial epididymitis.
Testis Biopsy
Indications
The indications for testis biopsy are detailed in Chapter 21. Briefly, testis biopsy is indicated in azoospermic men with testis of normal size and consistency, palpable vasa deferentia, and normal serum follicle-stimulating hormone (FSH) levels. Under these circumstances, biopsy will distinguish obstructive from nonobstructive azoospermia. In men with congenital absence of vasa and normal serum FSH levels, biopsy always reveals spermatogenesis (Goldstein and Schlossberg, 1988) and biopsy is not necessary before definitive sperm aspiration and IVF with ICSI. Diagnostic biopsy should usually be performed bilaterally irrespective of the size discrepancy of the two testes. Good spermatogenesis is sometimes found in small, firm testes, whereas biopsies of large, healthy testes may reveal maturation arrest.
The ability to achieve pregnancy with only a single testicular sperm has turned biopsy into a potentially therapeutic, as well as diagnostic, procedure. Even men with markedly elevated serum FSH levels and small, soft testes (meaning certain testicular failure) often harbor rare mature sperm in their testes. These sperm can be extracted using techniques described later (see Testicular Sperm Extraction) and employed for IVF with intracytoplasmic injection of testicular sperm.
The recently discovered heterogeneity of the testes of men with nonobstructive azoospermia, coupled with the ability of testicular sperm to acquire motility (Jow et al, 1993), has resulted in changes in the techniques of testis biopsy. Examination of fresh, unfixed tissue for the presence of sperm with tails and possible motility, as well as examination of multiple samples if sperm are not found initially, are now recommended. Furthermore, optimal care requires the availability, at the time of biopsy, of an andrology laboratory capable of processing and cryopreserving any sperm found at the time of biopsy.
Open Testis Biopsy—Microsurgical Technique
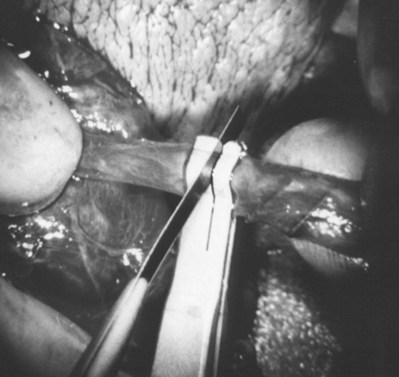
Open biopsy remains the gold standard because it provides an optimal amount of tissue both for accurate diagnosis and retrieval of sperm for IVF (Rosenlund et al, 1998; Schlegel, 1999; Dardashti et al, 2000). Open testis biopsy may be performed using either general, spinal, or local anesthetic. Although local anesthesia of just the skin and tunicas without a cord block is uncomfortable, local anesthesia with spermatic cord block can be effective and comfortable. However, there are limitations of the cord block. In animal studies, the incidence of accidental damage to the testicular artery during blind cord block is 5% (Goldstein et al, 1983). In addition, if there has been previous scrotal surgery with scar or adhesions and more extensive dissection and manipulation may be required, the author prefers to use general or spinal anesthetic.
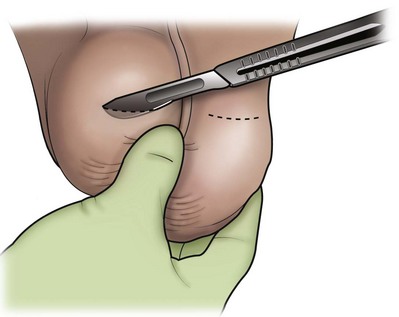
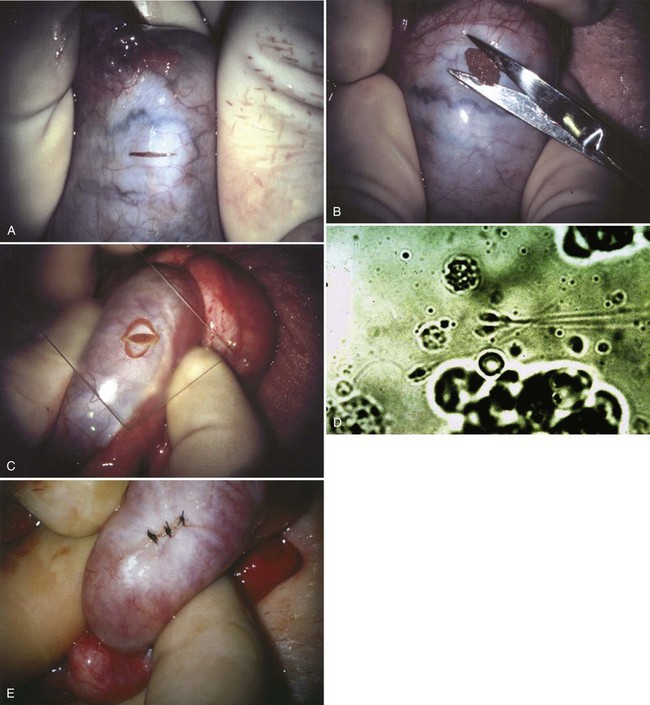
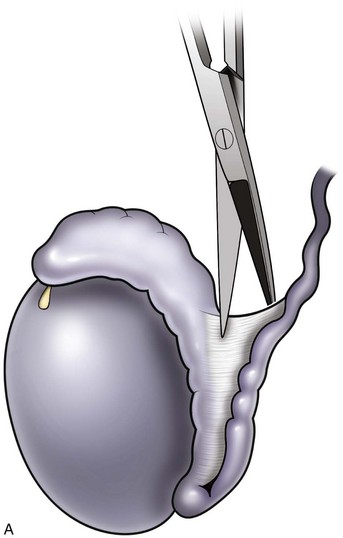
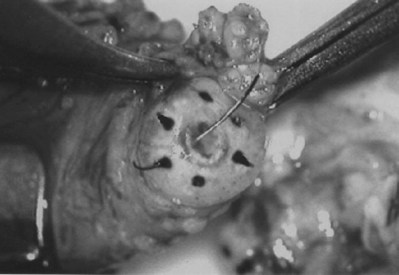
An assistant stretches the scrotal skin tightly over the anterior surface of the testis and confirms that the epididymis is posterior. Bilateral 1-cm transverse scrotal incisions provide good exposure with a minimum of scrotal bleeding (Fig. 22–1). Alternatively, a single vertical incision in the median raphe may be employed. The incision is carried through the skin and dartos muscle, and the tunica vaginalis is opened. If the anatomy is distorted from previous surgery, the epididymis cannot be clearly palpated posteriorly. If the tunica albuginea cannot be clearly identified, the incision should be enlarged and the testis delivered. The edges of the tunica vaginalis are held open with hemostats and any bleeding vessels are cauterized. Use of loupes or, better yet, the operating microscope allows ready identification of a spot on the tunica albuginea relatively free of visible surface vessels. The wound should be dry before incising the tunica albuginea to prevent saturation of the biopsy with blood. A 3- to 4-mm incision is made in the tunica albuginea with a 15-degree microknife (Fig. 22–2A). Small crossing vessels are cauterized with bipolar cautery and divided before excising a pea-sized sample of seminiferous tubules with razor-sharp iris scissors (Fig. 22–2B). When handling testis biopsy material for permanent fixation, avoid tissue handling in any way (including with forceps) because this may traumatize and distort the testicular architecture. The specimen is then deposited directly into Bouin, Zenker, or collidine-buffered glutaraldehyde solution. Formalin fixation results in distortion of testicular histology and should not be used for testis biopsy. A touch-prep is made by blotting the cut surface of the testis several times with a glass slide (Fig. 22–2C) and adding a drop of saline, lactated Ringer, or human tubal fluid with IVF medium and a cover slip. Examination under high power using a light microscope with or without phase contrast will reveal the presence of sperm with tails and allow assessment of motility (Fig. 22-2D). If no sperm are found in the touch prep, a second specimen may be cut for a wet squash prep. In this case the specimen is placed on a slide, a drop of saline is added, and the specimen is crushed under a coverslip (Jow et al, 1993). If no sperm are found, the tunica is closed with two to three interrupted sutures of 5-0 Vicryl (Fig. 22-2E) and another area is biopsied through the same skin incision. As described later in this chapter, use of an operating microscope providing 10× to 25× magnification may allow selective biopsy of larger seminiferous tubules more likely to contain sperm (Schlegel, 1999). If sperm are identified, the slide and additional tissue removed are sent for cryopreservation in the andrology laboratory. The location of the biopsy site where sperm were found is noted, and the tunica albuginea is closed with two to three interrupted sutures of 6-0 nylon. This assists identification of sites of spermatogenesis for future testicular sperm extraction for IVF/ICSI.
Percutaneous Testicular Aspiration
Testicular aspiration performed with a 23-gauge needle or angiocath sheath (Marmar) is probably less invasive and less painful than percutaneous biopsy. Although flow cytometric evaluation of this material can distinguish haploid from diploid cells and therefore confirm the presence or absence of late stages of spermatogenesis (Chan et al, 1984), direct wet examination of the aspirate for sperm and assessment of motility provide the most practical clinical information. Three or four aspirations can be performed until sperm are identified. In cases of obstructive azoospermia, these sperm can be used for IVF with ICSI (Craft et al, 1995) when sperm cannot be retrieved from the epididymis (see Testicular Sperm Extraction).
Complications of Testis Biopsy
Carefully performed, testis biopsy is associated with few complications (Schlegel and Su, 1997; Dardashti et al, 2000). The most serious complication associated with testis biopsy is inadvertent biopsy of the epididymis. If histologic evaluation of the biopsy material reveals epididymis with sperm within the epididymal tubule, obstruction of the epididymis at the site of the biopsy is certain. If, however, there are no sperm within the epididymal tubules, the patient is either obstructed above the level of the epididymal biopsy or has primary seminiferous tubular failure and no harm has been done.
Vasography
Indications
The absolute indications for vasography are as follows:
Relative indications for vasography are as follows:
Vasography should answer the questions:
If the testis biopsy reveals many sperm, consider the following:
Vasography with radiographic contrast media and intraoperative radiograph are rarely indicated. There is no need to perform vasography at the time of testis biopsy for azoospermia unless immediate reconstruction is planned and the touch/wet prep biopsy reveals mature sperm with tails. If not meticulously performed, vasography can cause stricture or even obstruction at the vasography site, which can complicate subsequent reconstruction (Howards et al, 1975a; Poore et al, 1997). In addition, vasography is of no value in making the diagnosis of epididymal obstruction and the majority of nonvasectomy-related obstructions are epididymal.
Technique of Vasography and Interpretation of Findings
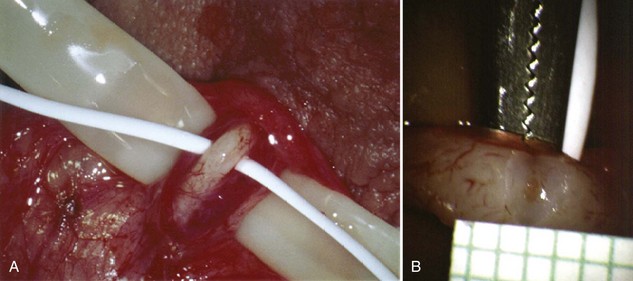
Inguinal hernia repair, particularly when performed in childhood, is known to be associated with vasal injury leading to obstruction. If there is no previous inguinal incision and the side of obstruction is unknown, the testis is delivered through a high vertical scrotal incision (see Scrotal later). The vas deferens is identified and isolated at the junction of the straight and convoluted portions of the vas deferens. Using an operating microscope and 10-power magnification, the vasal sheath is longitudinally incised and the vasal vessels carefully preserved (Fig. 22–3A).
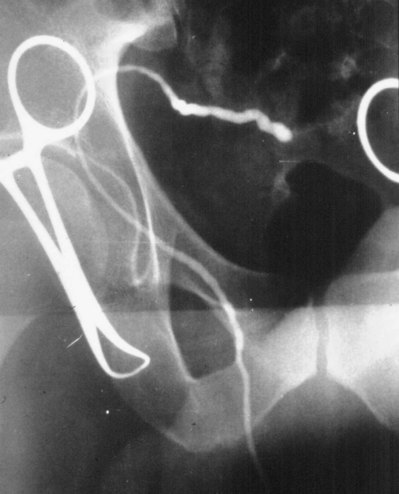
A clean segment of bare vas is delivered and surrounded with a vessel loop. A straight clamp is placed beneath the vas to act as a platform. Under 25-power magnification, a 15-degree microknife is used to hemitransect the vas until the lumen is revealed (Fig. 22–3B). Any fluid exuding from the lumen is placed on a slide, mixed with a drop of saline, and sealed with a coverslip for microscopic examination. If the vasal fluid is devoid of sperm with repeated sampling after milking the epididymis and convoluted vas, epididymal obstruction is present. The end of the vas toward the seminal vesicles is then cannulated with a 24-gauge angiocatheter sheath and injected with 1 mL of lactated Ringer solution with a 1-mL tuberculin syringe to confirm its patency (Fig. 22–4). If the Ringer passes easily, formal vasography is not necessary. If further proof of patency of the vas deferens is desired, 1 mL of 50% dilute indigo carmine may be injected and the bladder catheterized. The presence of blue/green dye in the urine confirms patency of the vas. Indigo carmine diluted 50/50 with lactated Ringer solution is preferred instead of methylene blue because, even at low concentrations, methylene blue kills sperm and renders them useless for cryopreservation or for immediate IVF/ICSI (Chang et al, 1998; Sheynkin et al, 1999b; Wood et al, 2003). If motile sperm are found in the vas, the testicular end should be gently barbotaged with 0.2 mL of human tubal fluid media and the fluid processed by the andrology laboratory for sperm cryopreservation for potential future use for IVF/ICSI. This should be done before injection with indigo carmine or x-ray contrast material (Sheynkin et al, 1999a).
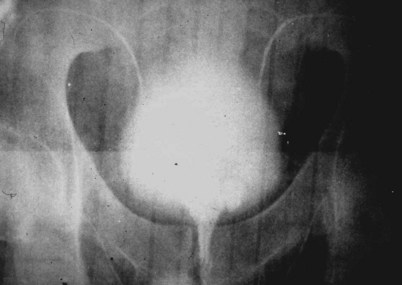
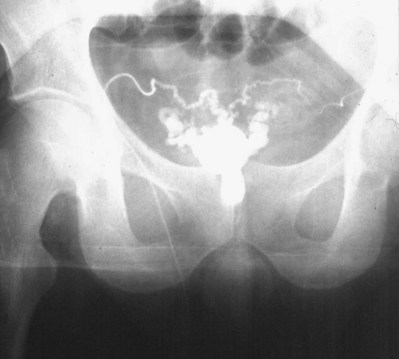
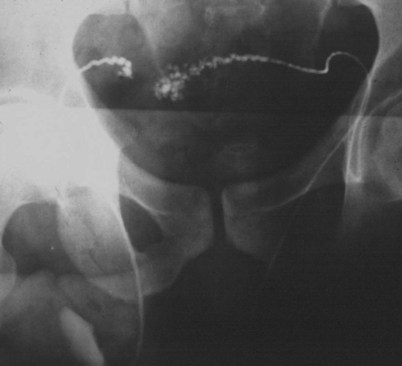
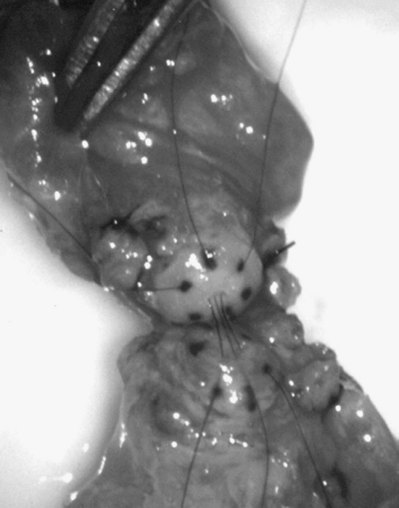
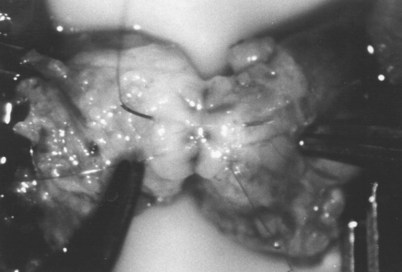
If a large amount of fluid is found in the vasal lumen and microscopic examination reveals the presence of sperm, the obstruction is toward the seminal vesicle end of the vas. In these cases the vas is usually markedly dilated. A 2-0 Proline suture can be passed toward the seminal vesicle end of the vas and a clamp placed on the Proline when the suture passes no farther. This is particularly useful for delineating the site of inguinal obstruction from prior groin surgery. If the obstruction is proximal to the inguinal scar, formal vasography is performed by passing a No. 3 whistle-tip ureteral catheter toward the seminal vesicle end of the vas. A 16-Fr Foley catheter is placed in the bladder, and the balloon is filled with 5 mL of air. Placing the balloon on gentle traction before vasography prevents reflux of contrast into the bladder, which can obscure detail (Fig. 22–5). The air-filled balloon also identifies the location of the bladder neck relative to any obstruction. After the vasa have been cannulated, vasograms are performed with the injection of 0.5 mL of water-soluble contrast media (Fig. 22–6). If vasography reveals obstruction at the site of the ejaculatory ducts (Fig. 22–7), indigo carmine is injected in both vasa to assist a transurethral resection (TUR) of the ejaculatory ducts (see Diagnosis later). If both vasa are visualized after injection of contrast into only one vas (Fig. 22–8), it means both vasa empty into a single cavity, usually a midline ejaculatory duct cyst.
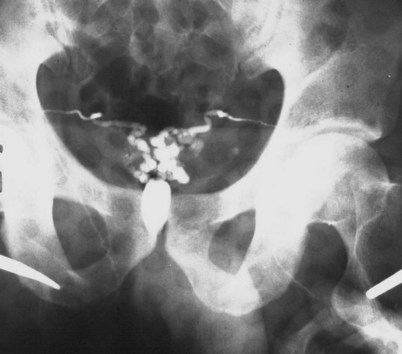
Figure 22–8 Both vasa are visualized after injection of contrast into one vas only, revealing distal obstruction.
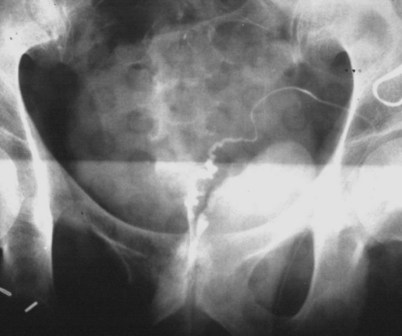
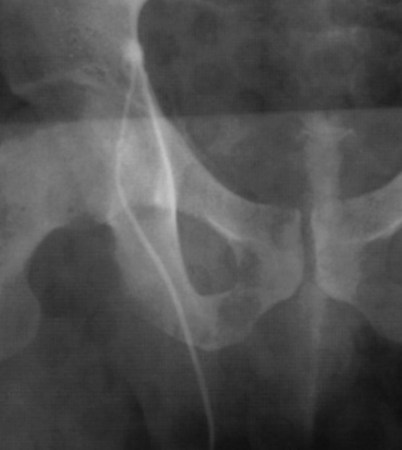
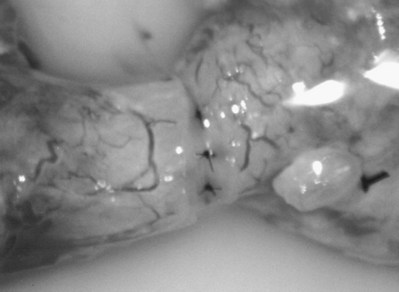
Vasography may reveal the vas deferens ending blindly, far from the ejaculatory ducts (Fig. 22–9). This finding indicates congenital partial absence of the vas deferens, and these patients should be tested for cystic fibrosis mutations (see Chapter 21). If this is found bilaterally (Fig. 22–10), reconstruction is impossible but vasal or epididymal sperm can be aspirated into standard laboratory pipettes (see Microsurgical Epididymal Sperm Aspiration Techniques later) and cryopreserved for future IVF with ICSI. If vasography reveals obstruction in the inguinal region (Fig. 22–11), either inguinal vasovasostomy or crossed transeptal vasovasostomy, using the contralateral unobstructed vas (see Crossed Vasovasostomy later), may be performed. The hemitransected vasography sites are carefully closed microsurgically using two or three interrupted 10-0 monofilament nylon for the mucosa and 9-0 for the muscularis and adventitia (see Microsurgical Multilayer Microdot Method later).
If the vasal fluid reveals no sperm and vasography confirms patency of the seminal vesicle end of the vas, the vas is completely transected and the seminal vesicle end is prepared for vasoepididymostomy (see Vasoepididymostomy later). If the vasal fluid reveals many sperm and vasography is normal, either retrograde ejaculation, lack of emission, or aperistalsis of the vas (Tiffany and Goldstein, 1985; Tillem and Mellinger, 1999) is the cause of the azoospermia.
Fine-Needle Vasography
Exposure of the vas in its straight portion may allow vasography to be performed with a fine needle, obviating the need for hemitransection of the vas. Dewire and Thomas (1995) employed a 30-gauge lymphangiogram needle attached to Silastic tubing. When the sensation of puncture of the lumen is detected, 50% water-soluble contrast is injected to confirm patency radiographically. This has proven to be a difficult technique for even experienced microsurgeons to master. Accurate evaluation of vasal fluid for sperm is difficult because it is so scant. If barbotage with saline or lactated Ringer solution reveals the presence of sperm, then epididymal obstruction has been excluded and contrast can be injected. Collection of vasal sperm for cryopreservation is difficult with this technique. Percutaneous vasography through the scrotal skin has been successfully performed in China (Li) using the same ringed percutaneous fixation clamp employed for the no-scalpel vasectomy. After fixation of the vas beneath the scrotal skin, the vas lumen is punctured with a 22-gauge sharp needle and cannulated with a 24-gauge blunt needle through which vasography is performed. This technique is even more difficult than the direct-vision technique with a fine needle.
Complications of Vasography
Stricture
Multiple attempts at percutaneous vasography using sharp needles can result in stricture or obstruction at the vasography site. Imprecise closure of a vasotomy can also result in stricture and obstruction (Howards et al, 1975b; Poore et al, 1997). Non-water-soluble contrast agents may also result in stricture and should not be employed for vasography.
Transrectal Vasography and Seminal Vesiculography
If transrectal ultrasound reveals markedly dilated seminal vesicles and/or a midline müllerian duct cyst in a man with obstructive azoospermia, transrectal aspiration followed by instillation of indigo carmine mixed with radiographic contrast is a useful diagnostic maneuver (Jarow, 1994; Katz et al, 1994; Riedenklau et al, 1995).
The same bowel prep and antibiotic coverage used for transrectal prostate biopsy is employed. The fine-needle aspirate is examined for sperm. If sperm are present it means at least one vas and epididymis are patent. One-half mL of indigo carmine is diluted with 1.5 mL of 50% water-soluble contrast and instilled. If a flat plate reveals a potentially resectable lesion, a TUR of the ejaculatory ducts is performed (see Transurethral Resection of the Ejaculatory Ducts later). Visualization of blue dye effluxing from the ejaculatory ducts or an unroofed cyst aids in determining the adequacy of the resection (Cornel et al, 1999).
Vasovasostomy
Introduction
The number of American men who undergo vasectomy has remained stable at about 500,000 per year, as has the divorce rate of 50%. Surveys suggest that 2% to 6% of vasectomized men will ultimately seek reversal. Furthermore, obstructive azoospermia can be the result of iatrogenic injuries to the vas deferens, usually from hernia repair, in 6% of azoospermic men (Sheynkin et al, 1998a; Shin et al, 2005).
Laboratory Tests
Surgical Approaches
Scrotal
Bilateral high vertical scrotal incisions provide the most direct access to the obstructed site in cases of vasectomy reversal. Length is usually a problem on the abdominal end but not on the testicular end. Mark the location of the external inguinal ring (Fig. 22–12). If the vasal gap is large or the vasectomy site is high, this incision can easily be extended toward the external ring. If the vasectomy site is low, it is easy to pull up the testicular end. This incision should be made at least 1 cm lateral to the base of the penis. The testis should be delivered with the tunica vaginalis left intact. This provides excellent exposure of the entire scrotal vas deferens and, if necessary, the epididymis.
Preparation of the Vasa
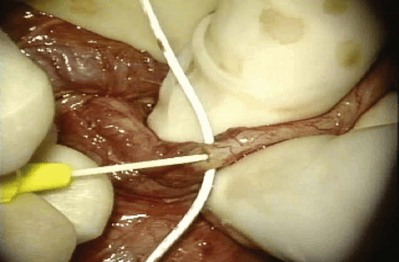
When large vasal gaps are present, a gauze-wrapped index finger is used to bluntly separate the cord structures from the vas. Blunt finger dissection through the external ring will free the vas to the internal inguinal ring if additional abdominal side length is necessary. These maneuvers will leave all the vasal vessels intact. When the vasal gap is extremely large, additional length can be achieved by dissecting the entire convoluted vas free of its attachments to the epididymal tunica (Fig. 22–13), allowing the testis to drop upside down. These maneuvers can provide an additional 4 to 6 cm of length. In order to maintain the integrity of the vasal vessels, this dissection is best performed using magnifying loupes or the operating microscope under low power. If the amount of vas removed is so large that even these measures fail to allow a tension-free anastomosis, the incision can be extended to the internal inguinal ring, the floor of the inguinal canal cut, and the vas rerouted under the floor, as in a difficult orchiopexy. An additional 4 to 6 cm of length can be obtained by dissecting the epididymis off of the testis from the vasoepididymal junction to the caput epididymis (Fig. 22–14A and B). The superior epididymal vessels are left intact and provide adequate blood supply to the testicular end of the vas. With this combination of maneuvers, up to 10 cm gaps can be bridged.
After the vasa have been freed, the testicular end of the vas is cut transversely. An ultrasharp knife drawn through a slotted 2-, 2- to 5-, or 3-mm diameter nerve holding clamp (Accurate Surgical and Scientific Instrument Corp., Westbury, NY) yields a perfect 90-degree cut (Fig. 22–15). The cut surface of the testicular end of the vas deferens is inspected using 15- to 25-power magnification. A healthy white mucosal ring that springs back immediately after gentle dilation should be seen. The muscularis should appear smooth and soft. A gritty-looking muscularis layer indicates the presence of scar/fibrotic tissues. The cut surface should look like a bull’s-eye with the three vasal layers distinctly visible. Healthy bleeding should be noted from both the cut edge of the mucosa and the surface of the muscularis. If the blood supply is poor or the muscularis is gritty, the vas is recut until healthy tissue is found. The vasal artery and vein are then clamped and ligated with 6-0 nylon. Small bleeders are controlled with a microbipolar forcep set at low power. Once a patent lumen has been established on the testicular end, the vas is milked and a clean glass slide is touched to its surface. The vasal fluid is immediately mixed with a drop or two of saline or lactated Ringer solution and preserved under a cover slip for microscope examination. The abdominal end of the vas deferens is prepared in a similar manner, and the lumen gently dilated with a microvessel dilator and cannulated with a 24-gauge angiocatheter sheath. Injection of saline or lactated Ringer solution confirms its patency. After the Ringer injection and a test dilation, the vas is recut to obtain a fresh surface. A minimum of instrumentation of the mucosa should be performed.
After preparation, the ends of the vasa are stabilized with a Microspike approximating clamp (Goldstein, 1985) to remove all tension before performing the anastomosis. Isolating the field through a slit in a rubber dam prevents microsutures from sticking to the surrounding tissue. A sterile tongue blade covered with a large Penrose drain is placed beneath the ends of the vasa to provide a platform on which to perform the anastomosis.
When to Perform Vasoepididymostomy
The gross appearance of fluid expressed from the testicular end of the vas is usually predictive of findings on microscopic examination (Table 22–2). If microscopic examination of the vasal fluid reveals the presence of sperm with tails, vasovasostomy is performed. If no fluid is found, a 24-gauge angiocatheter sheath is inserted into the lumen of the testicular end of the vas and barbotaged with 0.1 mL of saline while the convoluted vas is vigorously milked. The barbotage fluid is expressed onto a slide and examined. Men with large sperm granulomas often have virtually no dilation of the testicular end of the vas and little or no fluid initially; however, with barbotage and vigorous milking, sperm can invariably be found in this scant fluid. If there is no sperm granuloma and the vas is absolutely dry and spermless after multiple samples are examined, vasoepididymostomy is indicated. If the fluid expressed from the vas is found to be thick, white, water insoluble, and like toothpaste in quality, microscopic examination rarely reveals sperm. Under these circumstances, the tunica vaginalis is opened and the epididymis inspected. If clear evidence of obstruction is found (i.e., an epididymal sperm granuloma with dilated tubules above and collapsed tubules below), vasoepididymostomy is performed. When in doubt or if not experienced with vasoepididymostomy, vasovasostomy should be performed. However, only 15% of men with bilateral absence of sperm in the vasal fluid after barbotage and an intensive search will have sperm return to the ejaculate after vasovasostomy (Sheynkin et al, 2000).
Table 22–2 Relationship between Gross Appearance of Vasal Fluid and Microscopic Findings
| VASAL FLUID APPEARANCE | MOST COMMON FINDINGS ON MICROSCOPIC EXAMINATION | SURGICAL PROCEDURE INDICATED |
|---|---|---|
| Copious, crystal clear, watery | No sperm in fluid | Vasovasostomy |
| Copious, cloudy thin, water soluble | Usually sperm with tails | Vasovasostomy |
| Copious, creamy yellow, water soluble | Usually many sperm heads; occasionally sperm have short tails | Vasovasostomy |
| Copious, thick, white toothpaste-like, water insoluble | No sperm | Vasoepididymostomy |
| Scant white thin fluid | No sperm | Vasoepididymostomy |
| Dry spermless vas—no granuloma at vasectomy site | No sperm | Vasoepididymostomy |
| Scant fluid, granuloma present at vasectomy site | Barbotage fluid reveals sperm | Vasovasostomy |
Multiple Vasal Obstructions
If saline injection reveals that the abdominal end of the vas deferens is not patent, a 2-0 nylon or polypropylene suture is gently threaded into the vas lumen to determine the site of obstruction. If the obstruction is within 5 cm of the original vasectomy site, the abdominal end of the vas deferens may be dissected to this site and excised. The incision should then be extended inguinally to free the vas up extensively toward the internal inguinal ring. The testicular end then should also be freed up to the vasoepididymal junction. If the site of the second obstruction is so far from the vasectomy site that two vasovasostomies are necessary, a single crossed vasovasostomy should be performed to yield one good system (see Crossed Vasovasostomy earlier). If this is not possible, vasal or epididymal sperm is aspirated into micropipets and cryopreserved for future IVF with ICSI (see Sperm Retrieval Techniques later). Simultaneous vasovasostomies at two separate sites may lead to devascularization of the intervening segment with fibrosis and necrosis.
Varicocelectomy and Vasovasostomy
Microscopic varicocelectomy can assure preservation of the testicular artery in most cases. Deliberate preservation of the cremasteric veins provides venous return. In one series of 570 men presenting for vasectomy reversal, 19 had large varicoceles (20 left, 7 bilateral). Microsurgical varicocelectomy was performed at the same time as vasovasostomy. The cremasteric veins and the fine network of veins adherent to the testicular artery were left intact for venous return and to minimize the chances of injury to the testicular artery. Postoperatively 5 of 26 varicoceles recurred (22%) (Goldstein, 1995). This compares with a recurrence rate of less than 1% in 2700 varicocelectomies performed by the author in nonvasectomized men in whom the vasal vessels were intact and the cremasteric veins and periarterial venous network were ligated. However, Mullhall and colleagues performed a series of simultaneous microsurgical vasovasostomies and varicocelectomies without intentionally preserving the cremasteric and periarterial network. They reported a low recurrence rate and no cases of atrophy (Mulhall et al, 1997). Interestingly, the increase in recurrences when the cremasteric veins and periarterial venous network were left intact suggests that these veins contribute to a significant proportion of varicocele recurrences.
Anastomotic Techniques: Keys to Success
Sperm are highly antigenic and provoke an inflammatory reaction when they escape from the normally intact lining of the excurrent ducts of the male reproductive tract. Extravasated sperm adversely influence the success of vasovasostomy (Hagan and Coffey, 1977). Unlike blood vessel anastomoses, in which platelets and clotting factors seal the gaps between sutures, vasal and epididymal fluid contain no platelets or clotting factors, so the water-tightness of the anastomosis is entirely dependent on the mucosal sutures.
Microsurgical Multilayer Microdot Method
This method of vasovasostomy can handle lumina of markedly discrepant diameters in the straight or convoluted vas. The microdot technique ensures precise suture placement by exact mapping of each planned suture. The microdot method separates the planning from the placement (Goldstein et al, 1998). This allows focus on only one task at a time and results in substantially improved accuracy.
Immediately after drying the cut surface of the testicular end of vas with a Weck cell, a dot is made at 3 o’clock halfway between the mucosal ring and the outer edge of the muscle layer. A line is extended out from this dot to serve as a reference point. The second dot is made at 9 o’clock, and a line is extended from this dot as well. Additional dots are placed at 11, 1, 5, and 7 o’clock for a total of six. The abdominal end of the vas is marked in the same way to exactly match the testicular end (Fig. 22–16). Monofilament 10-0 nylon sutures, double-armed with 70-µm diameter taper-point needles bent into a fish hook configuration (available from Sharpoint and Ethicon), are used. Double-armed sutures allow inside-out placement (Fig. 22–17), eliminating the need for manipulation of the mucosa and the possibility of back-walling. If the mucosal rings are not sharply defined, stain the cut surfaces of the vasal ends with indigo carmine to highlight the mucosa (Sheynkin et al, 1999a). The anastomosis is begun with the placement of three 10-0 mucosal sutures anteriorly (Fig. 22–18). On the small abdominal side lumen, gently and momentarily dilate the lumen with a microvessel dilator just before placement of the sutures. For accurate mucosal approximation, include only a small amount of mucosa but one-third to one-half the muscle wall thickness. Include exactly the same amount of tissue in the bites on each side. The needle should exit through the center of each dot. After placement, tie the three mucosal sutures. Place two 9-0 monofilament nylon deep muscularis sutures exactly in between the previously placed mucosal sutures, just above, but not through the mucosa (Fig. 22–19) and then tie them. These sutures seal the gaps between the mucosal sutures without trauma to the mucosa from the larger 100-µm diameter cutting needle required to penetrate the tough vas muscularis and adventitia. Rotate the vas 180 degrees (Fig. 22–20) and place three additional 10-0 sutures through each microdot and then tie them to complete the mucosal portion of the anastomosis (Fig. 22–21). Just before tying the last mucosal suture, irrigate the lumen with heparinized Ringer solution to prevent the formation of clot in the lumen. After completion of the mucosal layer (Fig. 22–22), place four more 9-0 deep muscularis sutures exactly in between each mucosal suture, just above but not penetrating the mucosa. Place four to six 9-0 nylon interrupted sutures, between each muscular suture. This is a purely adventitial layer that covers the underlying mucosal suture. Finish the anastomosis by approximating the vasal sheath with six interrupted sutures of 8-0 nylon completely covering the anastomosis and relieving it of all tension (Fig. 22–23).
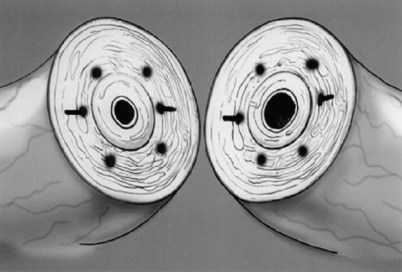
Figure 22–16 The abdominal end of the vas is marked in the same way to exactly match the testicular end.
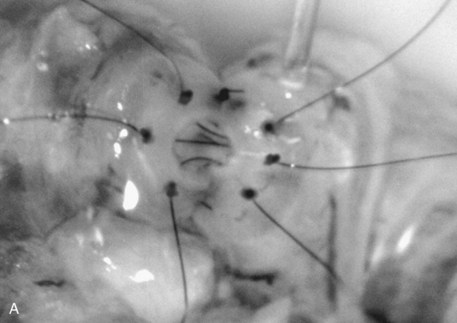
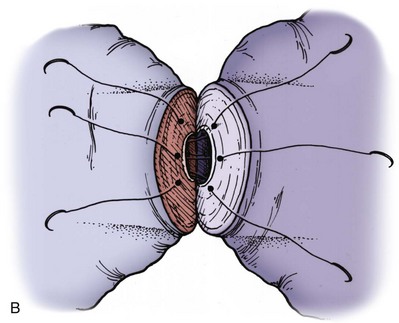
Figure 22–20 A and B, Rotate the vas 180 degrees and then place three more 10-0 sutures through the remaining microdots.
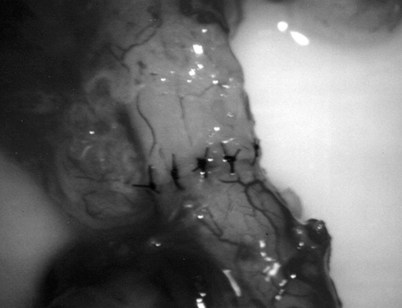
Figure 22–22 Additional sutures are placed in between the mucosal sutures, completing the anastomosis.
Anastomosis in the Convoluted Vas
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree