For patients with metastatic renal cancer, prognostic factors defined in systemic therapy clinical trials stratify patients into good, intermediate, and poor risk groups with median survival varying from 4 to 13 months. These same factors also stratify patients whose renal cancers were initially resected completely and who then developed subsequent metastatic disease. Metastasectomy performed in low-risk patients was significantly associated with enhanced survival when compared with low-risk patients not undergoing metastasectomy. Two randomized, prospective clinical trials demonstrated a modest survival advantage of approximately 6 months for patients undergoing cytoreductive nephrectomy followed by interferon alfa-2b. Once effective systemic agents are developed, both metastasectomy and cytoreductive nephrectomy will play greater roles in consolidating clinical responses.
There will be an estimated 54,390 new renal tumors and 13,010 deaths from renal cancer in the United States in 2008. Approximately 30% to 40% of patients with malignant renal cortical tumors will either present with or later develop metastatic disease. In metastatic renal cancer, surgical intervention may be performed alone or in combination with systemic therapy. Although the evolution of metastatic disease usually occurs within 2 years after the radical or partial nephrectomy, disease-free intervals of up to 30 years and metastases to unusual sites (eg, endocrine glands, digits) can cause diagnostic dilemmas. Unlike patients with renal cortical tumors detected incidentally during abdominal imaging obtained for other reasons, the vast majority of patients with metastatic renal tumors have large, locally advanced tumors often with regional nodal and or renal vein or inferior venal extension. Approximately 90% of the metastatic renal tumors are of the conventional clear cell histologic subtype. Unfortunately for metastatic renal cancer patients, systemic chemotherapy, cytokine therapy, and hormonal manipulations alone or in combination have low overall response rates (10%–15%) and are rarely associated with a complete remission. Median patient survival for metastatic renal cancer patients is in the range of 10 to 12 months. Rare spontaneous remissions of metastatic disease sites occur in less than 1% of cases and have been reported in both surgical series and the control arms of clinical trials with or without previous surgical intervention. New systemic chemotherapy agents, including multitargeted tyrosine kinase inhibitors (TKIs) (sunitinib, sorafenib) and mammalian target of rapamycin kinase inhibitors (temsirolimus, RAD001) are proving highly effective in clinical trials and are now actively being investigated in neoadjuvant and adjuvant surgical trials in patients with poor prognostic renal tumors.
Surgical intervention in any patient with metastatic renal cancer has one of two aims: (1) through metastasectomy, to render a patient clinically free of all sites of metastases, or (2), through cytoreductive nephrectomy to resect the primary tumor in the face of unresectable metastatic disease before the initiation of systemic therapy. The occasionally unpredictable natural history of renal cancer and varying patient selection criteria can make the interpretation of results from different centers difficult. Operative intervention in the face of metastatic renal cancer is controversial and is the subject of this review.
Metastasectomy
In 1939 Barney and Churchill first reported a patient who underwent nephrectomy and a resection of an isolated pulmonary metastasis for a renal cancer only to die 23 years later of coronary artery disease. Over the last 60 years, the surgical resection of limited metastatic disease (metastasectomy) was offered to patients and selectively performed in the absence of effective systemic therapies. The reported selection criteria for this aggressive surgical approach varied from study to study and reported significant prognostic factors included the site and number of metastatic deposits, completeness of resection, patient performance status, and the disease-free interval from treatment of the primary tumor to the diagnosis of metastatic disease. Complete resection of isolated metastases was associated with 5-year survival rates of between 35% and 60%. Despite successful resection of metastatic disease and associated patient survival, these studies lacked definitive proof that the surgical intervention itself, as opposed to patient selection factors and the natural history of renal cancer, led to the observed outcomes. Pogrebniak and colleagues reported 23 patients who underwent resection of pulmonary metastases from renal cell carcinoma (RCC), 15 of whom had previously been treated with IL-2–based immunotherapy. Patients with resectable lesions had a longer survival (mean 49 months) than those patients with unresectable lesions (mean 16 months). Furthermore, in this study, survival was not dependent upon the number of nodules removed. The investigators concluded that patients with metastatic RCC should be offered an operation if the likelihood that complete resection of all sites of disease were high. Favorable subgroups include those patients with a solitary site of metastases and disease-free interval to the development of metastases of greater than 1 year. It should be noted that occasionally sites of disease presumed to be metastatic RCC are instead secondary tumors (eg, pancreatic islet cell tumor) of either benign or malignant histology. This diagnostic dilemma may be addressed in the future with the further development of conventional clear cell specific immuno–positron emission tomographic scanning with 124-I cG250 scanning.
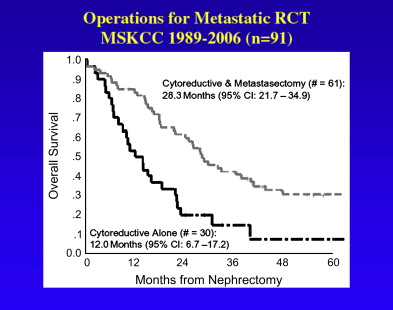
In a report from Memorial Sloan Kettering Cancer Center (MSKCC), prognostic factors associated with enhanced survival in 278 patients who underwent surgical metastasectomy included a disease-free interval of greater than 12 months (55% versus 9% 5-year overall survival), solitary versus multiple sites of metastases (54% versus 29% 5-year overall survival), and age younger than 60 years (49% versus 35% 5-year survival). Patient survival was longer when the solitary site of resection was lung (54% 5-year survival) compared with brain (18% 5-year survival). Twenty-nine percent of patients with completely resected multiple sites of metastases within a given organ survived 5 years, again suggesting that complete resection of all metastatic deposits was more important than the number metastatic deposits within a given site. Although the curative impact of metastasectomy is uncertain, operative intervention can also provide effective palliation for symptomatic metastatic disease to such sites as bone, brain, and adrenal gland. MSKCC investigators recently reported their experience with 61 patients who underwent nephrectomy followed by complete metastasectomy from 1989 to 2003. Of these patients, 59% had a Karnofsky performance status (KPS) greater than 90, 90% had conventional clear cell histology, and 62% had renal tumors that were greater than stage T2. Median survival was 30 months ( Fig. 1 ). A prospective and randomized clinical trial comparing metastasectomy to best standard systemic therapy could define the exact role of this approach.
Cytoreductive nephrectomy
The role of radical nephrectomy in patients with extensive metastatic renal cancer, when complete metastasectomy is not possible, has long been debated. Given the lack of effective systemic therapies and the unpredictable natural history of metastatic RCC, many oncologists referred patients to surgeons for resection of the primary tumor before cytokine-based therapy. In theory, cytoreductive radical nephrectomy is performed to remove a large, potentially immunosuppressive, tumor burden; to obtain accurate tumor histologic subtyping; and to prevent complications related to the primary tumor during systemic therapy. On rare occasions, a highly symptomatic tumor is removed for symptom palliation after the failure of conservative palliation measures (eg, unremitting gross hematuria or flank pain not relieved by conservative care or angioinfarction). Radical nephrectomy should not be done to induce spontaneous remission, a phenomena observed only in 4 of 474 patients (0.8%) treated with radical nephrectomy alone in a study from the Cleveland Clinic. Surgical mortality has been reported from 2% to 11% for patients with large primary renal tumors and metastatic disease. The possibility that the patient may not recover sufficiently to receive systemic immunotherapy after preparatory radical nephrectomy is of concern to surgeons and medical oncologists alike. In a study of 195 patients with metastatic RCC treated at the National Cancer Institute, 121 patients (62%) were eligible for high-dose IL-2 following cytoreductive nephrectomy, leading to a response rate of 18%. Yet, 38% of the patients in this series who underwent nephrectomy never received any immunotherapy either because of complications of nephrectomy or because of rapid clinical deterioration from disease progression. Some oncology groups recommend adjuvant radical nephrectomy only if initial systemic therapy was effective in initiating clinical regression of metastatic sites. This avoids the surgical morbidity.
Two randomized and prospective clinical trials have attempted to further define the role of cytoreductive nephrectomy in the treatment of metastatic renal cancer—one in the United States organized by the Southwest Oncology Group (SWOG), and the other in Europe organized by the European Organization Research and Treatment of Cancer (EORTC). Both used similar entry criteria comparing treatment for metastatic renal cancer with cytoreductive nephrectomy plus interferon alpha-2b versus interferon alpha-2b alone.
In the SWOG trial, between 1991 and 1998, 246 patients with metastatic renal cancer and with the tumor-bearing kidney in place from 80 participating institutions were randomly assigned to the two groups of 123 patients each. Eligible patients had a histologically confirmed diagnosis of metastatic RCC in tissue obtained by needle biopsy or aspiration of a least one measurable metastatic site or the primary tumor with metastases beyond the regional lymphatics involving a tumor of any size or any nodal status. Renal tumors with inferior vena caval extension below the hepatic veins were included. Patients were excluded if they received any prior systemic chemotherapy, hormonal therapy, cytokine therapy, biological response modifier therapy, or radiation therapy either to the primary tumor or any metastatic site. They were also excluded if the serum bilirubin was threefold higher than normal, if the serum creatinine was greater than 3.0 mg/dL, or if there was a history of significant cardiac arrhythmias or concomitant cancers less than 5 years before. Patients needed an Eastern Cooperative Oncology Group performance status of 0 or 1. Five patients were found to be ineligible because the pathologic diagnosis was incorrect (3 in the surgery arm and 2 in the interferon-alone arm). Of the 120 eligible patients analyzed in the nephrectomy-plus-interferon arm, 17 patients did not undergo the operation (7 refused, 5 were deemed medically unfit, 3 were unresectable, and 2 died before the operation). In the 98 patients evaluated for surgical complications, 1 patient died after operation for unresectable tumor with wound dehiscence and abdominal abscess with peritonitis, 2 patients had cardiac ischemia or infarction, 2 had postoperative infections, and 1 had hypotension. Sixteen patients had mild to moderate complications. In 76 of 98 patients (78%), no complications were reported. The mean hospitalization time was 8.2 days and the mean time to receive interferon alfa-2b was 19.9 days. Two eligible patients, 1 in each group, refused interferon therapy. One patient in the interferon group died of a treatment-related myocardial infarction and 23 patients (10 in surgical arm, 13 in interferon-only arm) had severe complications due to interferon. The therapeutic effect of interferon in both arms of the study was minimal for patients with measurable lesions at the time of treatment initiation with 3 partial responses in the surgery arm and 3 responses, 1 complete and 2 partial, in the interferon-alone arm. The extremely low rate of interferon responses in this study as opposed to other studies may be attributable to more stringent criteria for response used in the SWOG trial. At the time of the final analysis before publication, only 20 of the 241 (8%) eligible patients were alive. The patients who underwent cytoreductive nephrectomy and interferon alfa-2b had a significantly improved median survival of 11.1 months compared with 8.1 months for the interferon alfa-2b–alone group.
The EORTC study accrued 85 patients with metastatic renal cancer between 1995 and 1998 and, using a similar study design to the above-described SWOG protocol, randomly assigned patients to receive interferon alfa-2b alone or cytoreductive radical nephrectomy plus interferon alfa-2b. Two patients, 1 from each group, were ineligible and 4 patients in the cytoreductive surgery arm did not undergo operation. Forty of the 75 patients received at least 16 weeks of interferon alfa-2b therapy. Time to disease progression (5 versus 3 months) and median duration of survival (17 versus 7 months) significantly favored the patients in the cytoreductive nephrectomy–plus–interferon alfa-2b arm of the study. In this study, there were 5 complete remissions registered (1 in the interferon-alone arm and 4 in the cytoreductive nephrectomy arm). There were 6 postoperative complications, which included wound infection, pneumothorax, pneumonia, fever of unknown origin, cardiac arrhythmia, and cerebellar syndrome. Investigators from SWOG and EORTC later combined their data to analyze a total of 331 patients stratified at prerandomization by performance status (0 or 1), sites of metastases (lung or other), and disease measurability. The combined analysis of these two trials yielded a median survival of 13.6 months for the cytoreductive nephrectomy–plus–interferon alpha-2b arm versus 7.8 months for interferon alpha-2b alone. There was no difference in treatment effects according to prerandomization stratification factors. Unlike historical series, operative mortality in the combined experience was 1.5% (2 patients) and only 5.6% of patients did not proceed to interferon therapy. These improved surgical outcomes are likely a result of modern surgical techniques and contemporary perioperative care. In a recent update of 30 patients undergoing cytoreductive radical nephrectomy at MSKCC, median survival was 12 months, which is considerably less than the 30 months observed in the nephrectomy/metastasectomy patients (see Fig. 1 ).
While these trials show an apparent survival benefit of cytoreductive nephrectomy, such benefits may in part be attributed to referral patterns, surgical judgment, and patient selection, according to Bromwich and colleagues from Glasgow, United Kingdom. In their study of 94 patients with metastatic renal cancer evaluated from 1998 to 2001 for possible cytoreductive nephrectomy, 38 patients (40%) were considered inoperable and 36 patients (38%) were felt to have an Eastern Cooperative Oncology Group performance status of greater than 1. Cytoreductive radical nephrectomy was offered to 20 patients (22%) with a performance status of 0 or 1 and performed in 19. Of the 19, 13 patients began a course of immunotherapy (interferon) postoperatively. Seven patients had treatment-related toxicity necessitating withdrawal from the study and 4 patients had progressive cancer despite the interferon. Four patients were alive after cytoreductive nephrectomy (mean 8 months, range 3–16 months) and 15 have died of disease (mean time to death 9.5 months, range 3–28 months). The general impact of cytoreductive surgery and systemic therapy in this group of patients was minimal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








