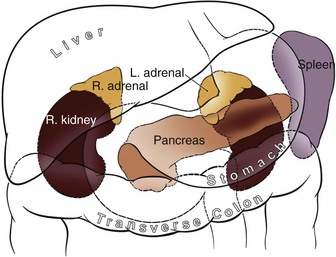
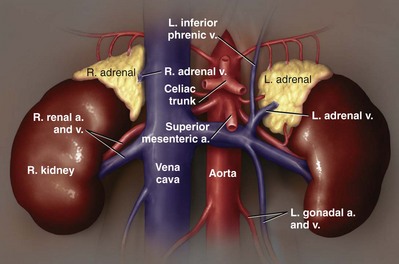
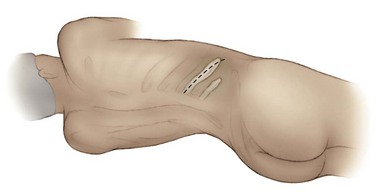
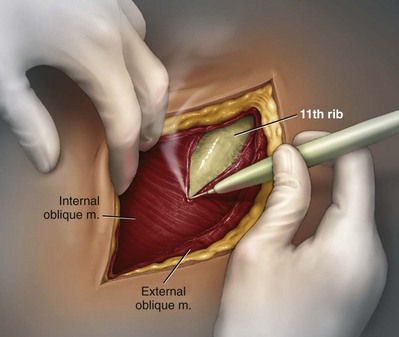
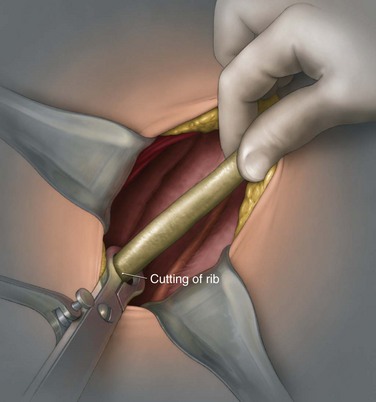
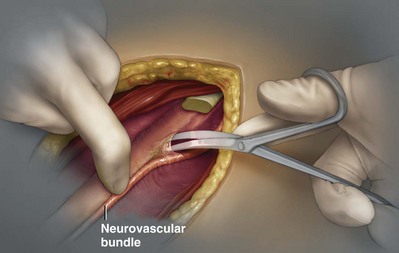
George K. Chow, MD, Michael L. Blute, Sr., MD Surgery of the adrenal gland consists of operative procedures to correct endocrine abnormalities or to treat malignant disease. As outlined in Chapter 57, various adrenal disorders can be identified and treated medically. When medical therapy is ineffective or does not exist for a particular adrenal disease, surgery becomes necessary. The first adrenalectomy was performed by Thornton in 1889 for a large tumor in a 36-year-old woman (Thornton, 1890). At the time, Thornton was unaware of the adrenal origin of the patient’s disease and removed the adrenal en bloc with the kidney. Sargent performed the first planned adrenalectomy in 1914 for a large adrenal adenoma. Charles Mayo (1927) performed the first flank adrenalectomy for pheochromocytoma in 1927. As was often the case in these early forays, Mayo was not aware of the exact nature of the pathologic process with which he was dealing and assumed that he was removing an aberrant retroperitoneal nerve. Young (1936) described the posterior approach using a “hockey stick” incision to access both adrenal glands simultaneously. The thoracoabdominal incision for management of large retroperitoneal masses was first described by Chute and colleagues (1949). In 1965, Turner-Warwick developed and described a supracostal transdiaphragmatic variation of Young’s posterior approach. Gagner performed the first laparoscopic adrenalectomy in 1991 (Gagner et al, 1992). He used a transperitoneal approach to gain access to the adrenal gland. Also in 1992, Gaur developed and described the first device for balloon dilation of the retroperitoneum. Retroperitoneal access has been developed for both flank (Gasman et al, 1998) and posterior (Baba et al, 1997) approaches. The left adrenal gland is bordered by the aorta medially, the stomach and body of the pancreas anteriorly, the kidney inferiorly, the spleen superiorly, and the diaphragm posteriorly. The left adrenal gland is often more elongated than the right adrenal gland and will lie in a more superomedial position to the kidney. This tends to place the gland closer to the left renal hilum, and these structures must be accounted for during dissection. The anatomic relationships of the adrenal glands are summarized in Fig. 58–1. The arterial blood supply to the adrenal gland can be variable but tends to derive from three major sources on each side. Each adrenal gland receives branches from its ipsilateral inferior phrenic artery (superior adrenal arteries), aorta (middle adrenal arteries), and renal artery (inferior adrenal arteries). The venous drainage is typically simple; a single adrenal vein drains into the vena cava directly on the right and into the left renal vein on the left. Accessory veins (5% to 10%) that drain into the right renal vein, right hepatic vein, or inferior phrenic vein can be present (Fig. 58–2). The clinical indications for adrenalectomy are summarized in Table 58–1. The pathophysiology, diagnosis, and treatment of these diseases are detailed in Chapter 57. Table 58–1 Clinical Indications for Adrenalectomy The current indications for open adrenalectomy are few but important. First, adrenal cortical carcinoma with radiographic evidence of extra-adrenal tumor invasion of adjacent organs may benefit from maximal surgical exposure. Similarly, the extension of adrenal vein tumor thrombus into the inferior vena cava necessitates a more invasive approach. Finally, in developing countries, the resources for laparoscopic surgery may be lacking, and the open approach will be preferred out of necessity. The choice of open surgical approach is dictated by the surgeon’s experience and the pathologic process in question. Table 58–2 summarizes the best surgical approach for a given adrenal disease. Table 58–2 Surgical Options (Open Adrenalectomy) From Vaughn ED. Adrenal surgery. In: Marshall F, editor. Operative urology. Philadelphia: WB Saunders; 1996. p. 222. Is open surgery indicated when there is a history of previous surgery close to the adrenal gland? The laparoscopic approach can be tailored to deal with past surgical history; a patient with previous transabdominal surgery could have a retroperitoneal laparoscopic procedure (Caddedu et al, 1999), and a history of previous open flank surgery could be addressed by performing a transperitoneal laparoscopic procedure. Furthermore, Gill and colleagues (2001) have reported their experience with a transthoracic laparoscopic approach that may be ideally suited for this situation. The thoracic cavity is entered thorascopically, the diaphragm is divided, and the adrenal gland is approached superiorly. Tumor size is a relative contraindication to laparoscopic surgery. In the initial experience of many laparoscopists, a cutoff of 5 or 6 cm was chosen because of the increased risk of treating an adrenal cortical carcinoma. Subsequently, ample empirical evidence has accumulated to suggest that specimen size is not necessarily a contraindication to laparoscopic adrenalectomy. MacGillivray and colleagues (2002) noted no difference in operative time, blood loss, complication rate, and hospital stay among 12 patients with large tumors (mean, 8.2 cm; range, 6 to 12 cm) and 36 patients with small tumors (mean, 2.5 cm; range, 0.4 to 5.6 cm). In contrast, Hobart and colleagues (2000) noted increased operative time (205 minutes vs. 158 minutes; P = .07), increased blood loss (400 mL vs. 113 mL; P = .009), higher complication rate (21.4% vs. 8.9%; P = .21), and higher open conversion rate (14.3% vs. 2.2%; P = .14) in comparing 14 large tumors (mean, 8 cm) with 45 small tumors (mean, 2.2 cm). However, Hobart did find that operative time, blood loss, hospital stay, narcotic use, and complication rate were lower with laparoscopic adrenalectomy than with traditional open adrenalectomy for large tumors. Although a higher morbidity could be expected with larger tumors, morbidity was still less than with open surgery. Conversion to open surgery is most often due to infiltrative adrenal cortical carcinoma. In the largest series, conversion occurred electively after initial laparoscopic exploration and not because of hemorrhage or other emergent causes. MacGillivray and colleagues (1996) concluded that preoperative computed tomographic scanning can identify those infiltrative tumors that are likely to be invasive carcinoma. MacGillivray and coworkers (2002) and Henry and colleagues (1999) recommend an upper size limit of 12 cm for laparoscopic adrenalectomy. However, keep in mind that computed tomographic scanning can underestimate specimen size by as much as 16% (Lau et al, 1999). With a worldwide epidemic of obesity, it is an increasingly common occurrence to operate on morbidly obese patients. Rather than obesity being a relative contraindication, laparoscopy may offer a less morbid alternative for adrenalectomy on obese patients. Comparing results between obese patients (body mass index greater than 30) who underwent laparoscopic adrenal or renal surgery and those who underwent open surgery, Fazeli-Matin and colleagues (1999) noted that patients who underwent laparoscopic surgery had fewer complications (18% vs. 47%; P = .21), less blood loss (50 mL vs. 300 mL; P = .03), less narcotic use (P = .01), and shorter hospital stay (<24 hours vs. 5.5 days; P = .01). However, these surgeries were performed by a veteran laparoscopist, and obesity may remain a relative contraindication for less experienced surgeons. Laparoscopic adrenalectomy on adrenal cortical carcinoma can be a daunting task. Adrenal carcinomas tend to be larger (>6 cm). Henry and colleagues (2002) noted that of 150 consecutive laparoscopic adrenalectomies, none of the smaller (<4 cm) tumors (N = 102) was malignant. In contrast, 12.5% of tumors larger than 4 cm (N = 48) were malignant. Using a 6-cm cutoff, Prager and coworkers (2004) noted that 21.2% of tumors larger than 6 cm were malignant versus 1.9% of lesions smaller than 6 cm. Also, the prospect of invasion by infiltrative lesions into surrounding structures can prohibit successful laparoscopic surgery. However, if computed tomography indicates that no local invasion is present and the lesion is not excessively large, laparoscopic adrenalectomy is possible. Known adrenal vein or vena caval involvement is an absolute contraindication to laparoscopic surgery. However, in one case report, Kim and colleagues (2004) described an intraoperatively discovered adrenal vein thrombus with a 7-cm adrenal mass. With use of atraumatic vascular clamps, the renal vein was entered, and the intact tumor thrombus was removed en bloc with the adrenalectomy specimen. Cancer control is dependent on adherence to the same oncologic principles as in open surgery. Wide local excision with periadrenal fat is necessary for good local control to be obtained. Adrenal cortical carcinoma tends to be an aggressive disease, and locoregional recurrence can develop as often as 60% (Vassilopoulou-Sellin and Schultz, 2001). Most reports of postoperative adrenal cortical carcinoma recurrence have been in case reports. Henry and colleagues (2002) published a summary of 12 cases of carcinoma recurrence in the medical literature. In 25% of the cases, the authors admitted to specimen disruption during the case. Potential errors in preoperative preparation are summarized in Table 58–3. Table 58–3 Errors in Preparation of Patients for Adrenal Surgery Modified from Vaughn ED. Complications of adrenal surgery. In: Taneja SS, Smith RB, Ehrlich RM, editors. Complications of urologic surgery: prevention and management. 3rd ed. Philadelphia: WB Saunders; 2001. p. 363. Open adrenalectomy can be performed through either a transperitoneal or retroperitoneal approach. The transperitoneal approaches include midline, subcostal, and thoracoabdominal. The retroperitoneal approaches include flank and posterior lumbodorsal. The advantages of the transperitoneal approaches are better exposure for larger tumors and excellent access to the great vessels and retroperitoneum. The main disadvantages are prolonged ileus and difficult exposure in morbidly obese patients. The retroperitoneal approach results in less ileus and may result in shorter hospital stays. There is a smaller operative field, and access to larger tumors and surrounding involved organs may be difficult. The best surgical approach for a given adrenal disease is indicated in Table 58–2. The patient is placed in flank position with the right side facing up (Fig. 58–3). The bed is placed in maximal flexion, and the kidney rest is deployed to accentuate the space between the costal margin and iliac crest. Palpation is used to identify the course of the 11th rib. The skin and fat overlying the 11th rib are incised, and the fascia and muscle overlying the rib are divided. (Fig. 58–4). Once the anterior surface of the rib is exposed, the anterior periosteum is cauterized, and the periosteal elevator is used to scrape it off the anterior rib surface. The edges of the periosteum on the superior and inferior aspects of the rib should now be visible. The periosteal elevator is used to develop a plane between the posterior rib surface and the posterior leaf of the periosteum. The Doyen instrument and surgical cautery are used to strip the periosteum off the rib from the tip of the rib back toward the paraspinal muscles. With the rib cutter, the 11th rib is excised (Fig. 58–5). A rongeur can be used to remove any sharp remnants on the rib stump. Cautery or bone wax can be used to render the marrow hemostatic. Next, the neurovascular bundle is identified and freed with sharp and blunt dissection to avoid injury during subsequent dissection and closure (Fig. 58–6). The lumbodorsal fascia is entered sharply with Metzenbaum scissors, and blunt dissection is used to dissect the peritoneum off the transverse fascia anteriorly. The flank muscles and their accompanying fasciae are divided anteriorly—the external oblique, internal oblique, and transverse abdominal. Next, the posterior muscle diaphragmatic attachments are divided with cautery. The pleura is sharply and bluntly dissected off the superior edge of the 12th rib. An incision is made along the course of the right 11th rib (Fig. 58–7). This is taken down to the periosteum, and the rib resection is performed in a manner similar to that described for flank incision. The diaphragm is dissected off the underlying peritoneum and liver. The peritoneum is then dissected off the Gerota fascia, which is retracted inferiorly. If a bilateral procedure is undertaken, a Finochietto retractor can be used to assist in bilateral exposure (Fig. 58–8
History
Surgical Anatomy
Clinical Indications and Selection of Patients
Clinical Indications
Indications for Open Surgery
DISEASE
APPROACH
Primary hyperaldosteronism
Posterior (left or right)
Modified posterior (right)
11th rib (left > right)
Posterior transthoracic
Cushing adenoma
11th rib (left or right)
Thoracoabdominal (large)
Posterior (small)
Cushing disease (bilateral hyperplasia)
Bilateral posterior
Bilateral 11th rib (alternating)
Adrenal carcinoma
Thoracoabdominal
11th rib
Transabdominal
Bilateral adrenal ablation
Bilateral posterior
Pheochromocytoma
Transabdominal chevron
Thoracoabdominal (large, usually right)
11th rib
Neuroblastoma
Transabdominal
11th rib
Past Surgical History
Size
Obesity
Adrenal Cortical Carcinoma
Preoperative Management
Primary Aldosteronism
Cushing Syndrome
Incidentalomas
Adrenal Carcinoma
Pheochromocytoma
Open Adrenalectomy
Flank Retroperitoneal Approach
Right Side
Lumbodorsal Posterior Approach
Right Side
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree