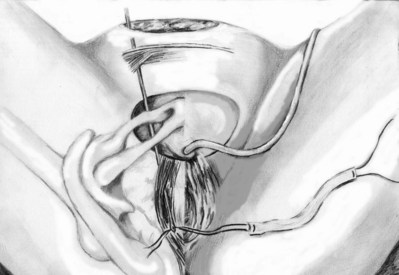
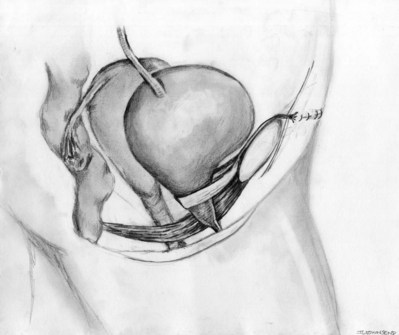
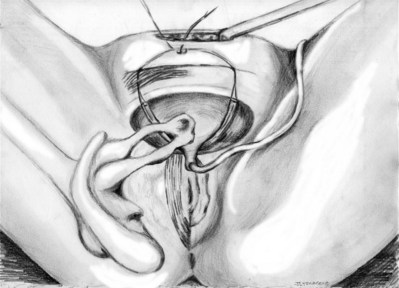
Roger R. Dmochowski, MD, FACS, Priya Padmanabhan, MD, MPH, Harriette Miles Scarpero, MD Urethral slings are currently the procedure of choice for the surgical correction of female stress urinary incontinence (SUI). A variety of materials (autologous, allograft, and synthetic) and techniques have been pursued for sling placement. The concept of slings for urethral support was first introduced in 1907 by Von Giordano as a gracilis muscle graft wrapping around the urethra (Blaivas, 1994). German surgeons were using slings fashioned from muscle and fascia for slings in children at the turn of the century (Goebell, 1910). The rectus abdominis muscle and fascia were first used by Frangenheim in 1914 and then adapted by Millin for use in recurrent SUI (Frangenheim, 1914; Millin, 1948). It was Aldridge who first understood the abdominal relationship between muscles and urethra and showed that an increase in abdominal pressure caused simultaneous compression of the urethra. He used rectus fascial slings in conjunction with vaginal plastic operations (Aldridge, 1942). Jeffcoate (1953) used the Aldridge technique on 40 women and reported an 86% cure rate. It was McGuire’s use of the pubovaginal sling (PVS) in patients who failed prior retropubic suspension and anterior colporrhaphy, with a cure rate of 91% that reintroduced this procedure (McGuire, 1978). This is the modern description of the PVS, with placement at the bladder neck, in an effort to correct urethral hypermobility and modify the pressure transmission invoked by intra-abdominal pressure changes (Blaivas and Olsson, 1988). In addition to the correction of urethral hypermobility, the PVS (a sling at the bladder neck as distinct from a midurethral sling) is also indicated for intrinsic sphincter deficiency (ISD) associated with urethral hypermobility, SUI presenting with concomitant cystoceles (Cross et al, 1997; Serels et al, 1999), and SUI associated with urethral diverticulum or urethral defects (e.g., urethrovaginal fistula) in which urethral reconstruction is required and in women with SUI and associated neurogenic conditions (Austin et al, 2001). In neurogenic patients, such as those with myelodysplasia, a PVS is indicated for SUI that occurs between clean intermittent catheterizations (once a thorough urodynamic study [UDS] of compliance and bladder capacity is completed). A PVS is used during reconstruction of proximal urethral loss secondary to trauma, or erosion (synthetic material or prolonged Foley catheter), or for interposition in repairs of urethrovaginal fistulae or urethral diverticula (Gormley et al, 1994; McGuire and O’Connell, 1995; Blaivas and Heritz, 1996; Leng and McGuire, 1998). A PVS is also indicated for recurrent SUI, especially after failed retropubic suspensions or prior midurethral sling placement (Beck et al, 1988; Petrou and Frank, 2001). The definition of ISD has evolved from a strict urodynamic criterion to a clinical diagnosis. In understanding surgical treatment it is essential to appreciate that all women with SUI have some component of ISD. The PVS is the gold standard for management of all forms of SUI. The PVS has less morbidity than older retropubic suspensions and a better long-term outcome than needle suspensions and urethral collagen injections. Using female Medicare beneficiary records, Anger and colleagues (2009) identified patterns in the surgical treatment of women with SUI in the United States from 1992 to 2001. By 2001, the PVS became the dominant anti-incontinence procedure, corresponding with a steady decline in needle suspensions and anterior urethropexies (Anger et al, 2009). The immobile urethra with poor intrinsic function has been classified as type III incontinence (Blaivas and Olsson, 1988) but is more commonly referred to as ISD. ISD is a complex condition with an imprecise definition and a large variability in measurement. The earliest measurements were by radiographic or endoscopic procedures, confirming an open proximal urethra and urethrovesical junction at rest (Blaivas and Olsson, 1988). Earlier methods for diagnosing ISD included the use of the supine stress test (empty and at capacity) (Lobel and Sand, 1996; Hsu et al, 1999), easy removal of inflated 8-Fr pediatric Foley balloon catheters as a positive office screening tool (Arya et al, 2001), color translabial sagittal ultrasonography for urethral vascularization measurement (Siracusano et al, 2002), ultrasound assessment of bladder neck funneling during straining (Huang and Yang, 2003), and translabial ultrasonography for measurement of the diameter of the urethra (Oliveira et al, 2006). In 2008, Oliveira and colleagues retrospectively reported lower opening detrusor pressure in stress incontinent women with ISD. Smith and Appell (2008) tried to determine whether voiding flow rates in women with symptomatic vaginal prolapse could predict ISD. Flow rates did not predict ISD (Hosker, 2009). ISD continues to be defined imprecisely by a low-pressure urethra based on urethral closure pressures and abdominal leak point pressures. A maximum urethral closure pressure (MUCP) of 20 cm H2O or less has been a determinant of ISD since McGuire’s (1981) research on failed SUI procedures. Although patients who experienced treatment failure were more likely to have an MUCP of 20 cm H2O or less, McGuire was unable to determine whether closure pressure was the cause or effect of the failures. Therefore, Sand and associates (1987) performed UDS on 86 women undergoing modified Burch colposuspensions. The women were divided into two groups: one with an MUCP of 20 cm H2O or less and one with an MUCP of more than 20 cm H2O. The group with lower urethral closure pressures had a 54% failure rate, compared with 18% in the group with higher urethral closure pressures. Sand and associates concluded that a modified Burch colposuspension was not appropriate in patients younger than age 50 years with an MUCP of 20 cm H2O or less. Also other authors have not used an MUCP of less than or equal to 20 cm H2O to define ISD (Clemons and La Sala, 2007; Fritel et al, 2008). Abdominal leak point pressure (ALPP) less than or equal to 60 cm H2O has been used as a determinant of ISD since videourodynamic analysis by McGuire and colleagues (1993). They found that women who leaked with low abdominal pressures (5 to 60 cm H2O) were most seriously incontinent and 75% had ISD on videourodynamics (proximal urethral pressure <10 cm H2O at 0.5 cm from the bladder neck or a nonfunctioning open sphincter with leakage). There was no correlation between ALPP and MUCP (Hosker, 2009). ISD is clinically relevant, but until the imprecision of diagnosis is addressed it cannot be utilized completely. Female SUI is due to a combination of urethral hypermobility and ISD. The female urethra lies under the pubic symphysis, and the pubourethral ligaments suspend the anterior urethral wall to the pubic arch. During Valsalva or other stress maneuvers the posterior wall of the urethra slides away from the anterior urethral wall and causes the bladder neck to open in cases of urethral hypermobility. The uneven pressure transmission combined with opening of the bladder neck (funneling) causes a loss of urine with stress maneuvers. ISD arises from defects within the urethra proper, so that the urethral sphincter is unable to coapt and generate enough resting urethral closing pressure to store urine in the bladder. The female urethra is composed of four separate tissue layers that assist in keeping it closed. It is compression from the middle muscular coat that helps to maintain the resting urethral closure mechanism and the outer seromuscular layer that augments this closing pressure. In normal circumstances the resting urethral closing pressure of the internal sphincter exceeds the resting or Valsalva pressure exerted by the bladder. Additionally, the fast-twitch fibers of the external sphincter allow for the sudden voluntary guarding reflex and the slow-twitch fibers provide continuous passive control by the involuntary guarding reflex. In addition to these structures the integrity of the pelvic diaphragm is dependent on the levator ani for continence control. The urethropelvic ligament and pubocervical fascia also provide needed support to the bladder neck and undersurface of the bladder, respectively, to prevent SUI (Vasavada and Rackley, 2011). The PVS is positioned at the bladder neck to provide urethral compression without obstruction during times of increased intra-abdominal pressure. The ultimate goal is to provide adequate urethral coaptation to increase urethral responsiveness to abdominal pressure. This must be balanced with the risks of ischemia, retention, and erosion from unnecessary tension. Aldridge’s (1942) earliest anatomic descriptions had fascia attached at the medial aspect of the rectus limiting sling mobility and provided no method for avoiding excess tension, resulting in outlet obstruction. The sling was often too short to pass under the urethra. McGuire (1978) modified this technique with a strip of rectus fascia 1 × 12 cm in length, which remained attached laterally on one side. In this technique there was also no way to adjust the tension with one side attached. The modifications by Blaivas and Jacobs (1991) with a free graft of rectus fascia whose tension could be adjusted (by pulling up and tying the sutures at each end of the free graft) are the present concept of the PVS. This allows for a shorter fascial sling. The key to the length of the fascial sling is its method of providing support. It is the incorporation of the sling into the endopelvic fascia and subsequent fibrosis, and not entry into the retropubic space, that prevents SUI. The width (2 to 3 cm) of the PVS ensures that there is sufficient support to provide the needed urethral compression and a cross-sectional area adequate to avoid the formation of a narrow constricting band. The choice of material in PVS requires material longevity and durability to allow for a strong sling scaffolding. The material should be incorporated and remain intact, with limited tissue reaction. Various substances have been utilized for construction of a PVS—autologous, allograft, xenograft, or synthetic materials. The ideal material provides long-lasting suburethral support with minimal complications. Ideally, implanted material should be incorporated into the host with minimal tissue reaction. In reality, most materials promote organized fibrosis, reinforcing the sphincteric mechanism through improved suburethral support. Theoretically, a greater degree of fibrosis leads to better clinical results (Bidmead and Cardozo, 2000; Woodruff et al, 2008). Yet, inflammatory infiltration leads to rapid sling material degradation and possible tissue destruction with erosion (Bidmead and Cardozo, 2000). Although there is complete biocompatibility of the autologous sling and negligible urethral erosion, biologic and synthetic graft materials have been increasingly used to decrease operative time, morbidity, pain, and hospital stay (Niknejad et al, 2002). Outcomes of these materials are discussed later. The most commonly used autologous materials include rectus fascia and fascia lata. Rectus fascia is harvested through a suprapubic Pfannenstiel incision. FitzGerald and associates (2000) reported that after sling placement, rectus fascial grafts undergo extensive remodeling with increased fibroblasts and connective tissue on biopsy specimens. Yet, histologic comparison of PVS grafts noted the greatest degree of host fibroblast infiltration and neovascularization in autologous materials with minimal inflammatory or foreign body reaction. The fascial graft changes were consistently intact with a small amount of sling degradation at explantation up to 65 months after placement (Woodruff et al, 2008). The rectus fascia harvest site may be scarred and thickened after prior operations, but this does not compromise its utility for PVS placement. The benefits of autologous tissue include the lack of tissue reaction and negligible urethral erosion (Webster and Gerridzen, 2003). Disadvantages include increased operative time and hospital stay, relative increase in postoperative pain, and suprapubic tissue seroma formation and hernia formation in a rectus fascial PVS (Gomelsky et al, 2003). Fascia lata is the other commonly utilized autologous material for a PVS. It is harvested from the thigh and has similar properties to rectus fascia (Beck et al, 1988; Latini et al, 2004). Methods for harvest are discussed in the section on operative procedure. Like rectus fascia, fascia lata is completely biocompatibility and is associated with minimal tissue reaction. The recovery time may be less, and there is no risk of future abdominal hernia formation, unlike rectus fascia. Yet, fascia lata requires repositioning of the patient, increased operative time, and operating in an area unfamiliar to urologists (Govier et al, 1997). Wheatcroft and colleagues (1997) reported 67% of their patients had pain on walking for 1 week after surgery. Latini and coworkers (2004) only reported 7% of patients with pain at incision site 1 week postoperatively after using a Crawford fascial stripper. Debility from a thigh hernia has also been reported. Another autologous material, vaginal wall, has also been used. Raz and associates (1989) described use of in-situ vaginal wall for autologous sling material. This tissue may lack sufficient tensile strength, and there is a risk of epithelial inclusion cyst formation and possible vaginal shortening. Lack of retropubic space dissection may militate against overall efficacy of this procedural variety (Raz et al, 1989; Ghoniem and Hassouna, 1998; Loughlin, 1998; Appell, 2000). Biologic and synthetic graft materials have been increasingly used to decrease operative time, morbidity, pain, and hospital stay. Cadaveric allografts have been used in many nonurologic surgical arenas (e.g., orthopedics, neurosurgery) and eventually adopted for SUI. Allograft slings are currently derived from either cadaveric fascia lata or acellular human dermis. After harvest the allografts are processed by solvent dehydration or by lyophilization (freeze-drying) to remove genetic material and to prevent the transmission of infectious agents. Secondary sterilization may also be achieved by gamma radiation (Gomelsky et al, 2003). Histologic analysis reveals cadaveric dermis to have little host fibroblast infiltration and little neovascularity, particularly in central aspects of the graft. Additionally, inconsistencies were found within the materials grossly, with specimens exhibiting significant thinning and degradation of the graft, disrupting the sling scaffold (Woodruff et al, 2008). No specific allograft has shown a clinical advantage in use. Acellular dermis rehydrates in 0.9% saline quicker than does cadaveric fascia lata (5 minutes vs. 15 to 30 minutes), but each type is pliable, easy to use, and available in a variety of sizes (Gomelsky et al, 2003). Biomechanical studies have shown that solvent-dehydrated cadaveric fascia lata and acellular dermis have a higher maximal load failure compared with freeze-dried cadaveric fascia lata (Hinton et al, 1992; Lemer et al, 1999). Lemer and associates (1999) prospectively studied the maximum load failure and stiffness of rectus fascia versus freeze-dried fascia versus solvent-dehydrated fascia and cadaveric dermal grafts. The mean values for maximum load to failure, maximum load-graft width, and stiffness were all significantly lower for the freeze-dried fascia lata group compared with the autologous, solvent-dehydrated, and dermal graft groups. The authors believed that ice crystal formation characteristics of tissue freezing may disrupt the collagen matrices, yielding poor strength properties. Dermal grafts differ from fascial allografts because they are derived from skin that is processed to eliminate the epidermis and all immunogenic cellular elements. Dermal grafts provide a protein matrix that serves as a collagen scaffold for the host’s own cellular matrix (Lemer et al, 1999). Although biomaterials were thought to be a good choice for increased biocompatibility, lower risk of erosion, and lack of response to hormonal stimuli, allografts raise the concern of potential transfer of disease, including human immunodeficiency virus (HIV), hepatitis, and Creutzfeldt-Jacob prion infection. There has been one documented case of HIV transmission from a tissue transplant since the onset of screening in 1985. The estimated risk of acquiring tissue from a properly screened donor infected with HIV is 1 in 1,667,600 (Gallantine and Cespedes, 2002). A few cases of Creutzfeld-Jakob disease (CJD) have been reported after transplantation of cadaveric dura or corneas; however, skin obtained from animals infected with prions has demonstrated no detectable infectious particles. Currently, the theoretical risk of developing CJD from a non-neural allograft is 1 in 3.5 million. No cases of hepatitis or CJD have ever been attributed to the use of processed cadaveric fascia or dermis (Amundsen et al, 2000b; Gallantine and Cespedes, 2002). Within the musculoskeletal tissue transplantation literature, two cases of hepatitis transmission have been reported. The first refers to one tissue donor (cancellous chips) who transmitted HIV, hepatitis B virus, and human T-lymphotrophic virus. These transmissions all occurred before the implementation of extensive donor screening for viruses and bacteria or the availability of validate serologic tests (or both) (Shutkin, 1954). In June 2002, the Centers for Disease Control and Prevention (CDC) reported a case of hepatitis C transmission from minimally processed, cryopreserved patellar tendon allograft. Donor screening was performed during the window period for hepatitis C virus (HCV). Retest of the donor sample with HCV RNA testing confirmed the donor as the source once the recipient reported HCV infection 1 year after transplantation (CDC, 2003; Vangsness, 2006). Despite the low risk of disease transmission, human DNA has been detected in various allograft materials (Choe and Bell, 2001; Hathaway and Choe, 2002). The clinical significance of this is unknown. The theoretical risk of developing hepatitis from allograft graft material is unknown. Xenografts have been utilized since the 1980s (Descurtins and Buchmann, 1982; Iosif, 1987) owing to their immediate accessibility and use without morbidity. Porcine and bovine xenografts have been used as sling material with decreasing popularity in recent years. The forms of xenograft available for use are porcine dermis or small intestinal submucosa (SIS) and bovine pericardium. Modern processing techniques using diisocyanate to remove genetic material have made porcine safer and more pliable, yet there is significant loss of tensile strength after implantation in a 12-week rabbit model (Dora et al, 2004). Histopathologic analysis has shown porcine SIS to contain growth factors that may reduce significant host-graft immunologic reaction and result in less tissue scarring (Wiedemann and Otto, 2004). Although a majority of data support SIS as nonimmunogenic, animal studies by Thiel and colleagues (2005) suggest that an intense inflammatory reaction 30 to 90 days after subcutaneous implantation occurs. In a report by Kalota (2004), 6 of 18 (33%) patients experienced postoperative inflammation after a PVS procedure. Konig and coworkers (2004) reported a single case of postoperative inflammation with abscess formation. Ho and colleagues (2004) reported a similar reaction in 6 of 10 patients. All of the patients presented with pain and erythema at the abdominal incision, and 2 developed abscesses. Five of the 6 patients with inflammatory responses are currently dry. All were treated conservatively, except 1 patient who required abscess drainage. The etiology is unknown but is likely due to a foreign body reaction from the multilayered (8-ply) SIS material, a reactive manufacturing ingredient, or a tendency for suprapubic fat to produce an inflammatory reaction (John et al, 2008). Kubricht and colleagues (2001) have shown that porcine SIS has less tensile strength than cadaveric fascia lata (Kubricht et al, 2001). Bovine pericardium is available in a preparation either cross-linked with glutaraldehyde or as a non–cross-linked acellular matrix (Gomelsky et al, 2003). Histopathologic comparison of sling materials revealed xenograft (porcine dermis) to have no host fibroblast infiltration, no inflammatory reaction, and no foreign body reaction. Xenograft had the highest propensity to encapsulate. A capsule formed around the porcine dermis, isolating the graft from the periurethral tissue. The graft was described as appearing similar to its original appearance at time of implantation (Woodruff et al, 2008). In 1959, Francis Usher introduced the first synthetic biomaterial—polyethylene mesh—for use in hernia surgery. In the decades since there has been a transition to polypropylene and the introduction of additional synthetic materials (Amid, 1997). The first synthetic sling, made of nylon, was introduced in 1953 (Kraatz, 1953). The addition of synthetic material for use in PVS surgery brought obvious advantages: unlimited supply of artificial graft material in endless sizes and shapes, consistency in quality, and elimination of harvest site and decreased associated operative time. As compared with absorbable biomaterials, synthetic materials are more uniform, more consistent, and more durable. Additionally, artificial graft materials are sterile, biocompatible, noncarcinogenic, and free of biomaterials (Niknejad et al, 2002). On histopathologic comparison, synthetic materials demonstrated the greatest amount of fibroblast ingrowth and tissue ingrowth into the specimen. There is no degradation or disruption of the graft, and the mesh is completely infiltrated by the viable host tissue. Microscopically, the synthetic material has large amounts of fibroblasts and foreign body reaction characterized by giant cells and occasional microcalcification. This foreign body reaction is not visible grossly by graft disruption, and the graft was completely infiltrated by host tissue (Woodruff et al, 2008). Artificial graft materials do have potential drawbacks, including graft infection, genitourinary erosion, or vaginal extrusion. The chemical and physical properties of each artificial material and patient characteristics determine how the sling is incorporated into the surrounding tissue and its susceptibility to infection, rejection, erosion, or extrusion. The susceptibility to infection in multifilament fibers is proportional to the porosity and the pore size of the materials (Amid, 1997; Niknejad et al, 2002). Tightly woven mesh provides a safe harbor for small bacteria, excluding macrophages and polymorphonuclear leukocytes. Loosely woven mesh allows tissue ingrowth and neovascularization, without limiting cellular access. Tissue bonding to the mesh strengthens and supports the repair. A tightly woven and large-diameter filament mesh will tend to exhibit increased stiffness or decreased pliability, contributing to migration, extrusion, or erosion. The classification by Amid (1997) used for synthetic materials in hernia surgery may be practically applied to urology as well (Table 73-1). The most frequently used materials are grouped into four types. Type I are totally macroporous prostheses (Atrium, Trelex, Marlex, Prolene) containing pores larger than 75 µm, which is the pore size for admission of macrophages, fibroblasts, blood vessels, and collagen fibers (White et al, 1981; Bobyn et al, 1982; White, 1988). Type II includes totally microporous prostheses (polytetrafluoroethylene [PTFE]: GORE-TEX, Surgical Membrane, and Dualmesh) containing pores less than 10 µm in at least one of their dimensions. Type III includes a macroporous prosthesis with multifilamentous or microporous components (PTFE: Teflon; braided Dacron mesh: Mersilene; braided polypropylene mesh: Surgipro; and perforate PTFE patch: MycroMesh). Lastly, type IV includes biomaterials with submicronic pore size (Silastic, Cellgard (polypropylene sheeting). Type IV is not appropriate for hernia surgery unless used in combination with type I (Amid, 1992). The most commonly utilized synthetic material for a PVS is polypropylene mesh (Table 73–2). It is composed of loosely woven strands of synthetic material, with a pore size greater than 80 µm, permitting passage of macrophages that may allow better host tissue ingrowth compared with the smoother, more tightly woven counterparts (Kobashi et al, 2005). This represents type I among the Amid classification. In fact, Amid (1997) concluded that the risk of infection and seroma formation was avoided by utilization of type I prostheses. Table 73–1 Amid Classification for Synthetic Materials PTFE, polytetrafluoroethylene. Adapted from Amid PK. Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia 1997;1:15–21. Table 73–2 Synthetic Sling Materials Adapted from Niknejad K, Plzak LS, Staskin DR, Loughlin KR. Autologous and synthetic urethral slings for female incontinence. Urol Clin North Am 2002;29:597–611. Historically, sling techniques have changed to limit the associated morbid complications. Synthetic material is no longer utilized in a PVS graft to pull the bladder neck into a high retropubic position owing to high erosion rates (see section on complications). Instead, newer approaches position a sling at the midurethra (see later) (Niknejad et al, 2002). Before performing a PVS, a thorough history and physical examination is performed. Key points in the history should include preoperative voiding status and symptoms. Specifically, the relationship between stress maneuvers (i.e., cough, sneeze, laugh, weight bearing, exercise) and related incontinence should be elucidated. It is important to identify preoperative storage symptoms, such as frequency, urgency, and urinary urgency incontinence (UUI), because these symptoms may not resolve after a sling procedure. A complete history of all prior vaginal and abdominal operations should include graft material placement, prior urethrolysis, and/or prolapse surgery. A history of prior irradiation is useful because this may compromise the quality of a rectus fascial graft. Physical examination should include an abdominal examination and complete pelvic examination to assess urethral hypermobility, quality of vaginal epithelium, extrusion of previously placed graft material, and prolapse. Cystocele and other forms of prolapse should be assessed, because they may lead to symptomatology and be a source of future obstruction. Special attention should be paid to the anterior vaginal compartment to determine if coexistent prolapse is present. The authors recommend UDS on all women undergoing surgical intervention for SUI, with or without simultaneous prolapse correction. UDS is recommended by the International Consultation on Incontinence (ICI) before undergoing surgical procedures to obtain a more accurate preoperative diagnosis (Griffiths et al, 2005). Knowing the urodynamic abnormalities responsible for urinary manifestations is essential to directing treatment at the underlying pathophysiology. The American Urological Association (AUA) recognizes uroflow, cystometry, leak point pressures, sphincter electromyography, and radiographic visualization as five modalities useful in understanding bladder storage and emptying. Implicitly, diagnosing impaired detrusor contractility or obstruction requires Pdet/uroflow, and cystometry is necessary for identifying detrusor overactivity (DO) (Rutman and Blaivas, 2007). A cough stress test may demonstrate SUI, but it provides no information regarding a patient’s bladder storage and emptying characteristics. This information is essential for tailoring the appropriate treatment plan. “The bladder is an unreliable witness,” because symptoms are not predictive of diagnosis. UDS may aid the clinician in detection of preoperative DO, preoperative abnormal voiding patterns (high postmicturition residual, obstructed flow), and de novo DO (Digesu et al, 2009). Cundiff and colleagues (1997) studied 535 incontinent women and concluded that pure symptoms identified less than half of the patients with urodynamic SUI, recommending UDS for all patients considering surgical intervention. UDS should be performed with and without a pessary if prolapse is present. In the case of fascia lata harvesting, the SCDs are placed below the patient’s patella on the harvest side. The knee is elevated and supported with a 1-L bag of intravenous fluid or an appropriate cushion or pad. The involved extremity is internally rotated at the hip and secured to the table using 3-inch tape, below the operative site. The thigh is prepped and draped to expose the anterolateral aspect of the thigh from the greater trochanter to distal to the patella. The greater trochanter and lateral femoral condyle of the femur are identified and marked. These landmarks mark the proximal and distal attachments of the fascia lata (Dwyer and Kreder, 2008). A 6- to 7-cm Pfannenstiel incision is made approximately 2 cm above the pubic symphysis and carried down to the rectus fascia. A 2-cm wide by 8-cm long graft is marked out in the rectus fascia. The premarked graft is harvested out of the rectus fascia. With the use of Allis clamps the edges of the free fascia are freed from the underlying rectus muscle with bovie cautery to aid in a tension-free closure (Fig. 73–1A). The fascia is closed with a running 1 polydioxanone (PDS) suture or other alternative suture. The graft is placed in 0.9% normal saline solution after harvest. On a sterile side table, the overlying fat and perifascial tissue are cleaned off of the graft. A size 1 polydioxanone suture is utilized (perpendicular to sling fibers) at each end of the sling to run across the full width of the sling and tied down. Sutures are left long, and the graft is placed in the 0.9% normal saline until needed. For fascia lata harvest, a 3-cm longitudinal incision is marked beginning just above the patella over the iliotibial band (see Fig. 73-1B). Dissection is carried down to the level of the fascia lata, where two parallel incisions are made longitudinally in the fascia lata, 2 cm apart. The autologous fascia is bluntly lifted off the underlying muscle and clamped as far distally as possible with a right-angle clamp (3 to 4 cm) and transected, allowing one free end. The free end is secured with a 1-0 polypropylene, polydioxanone, or polyglactin suture, and the proximal fascia lata is lifted off the muscle belly with a thin, malleable retractor. The fascia lata is separated from both the adipose tissue and the muscle fibers by passing the retractor superficial and deep to the fascia lata. With the free distal end under tension, a Crawford fascial stripper is used to extend the fascial incision proximally and divide it before removal. Classically, the fascial strip was 20 × 2 cm in dimension; however, now shorter lengths (8 cm) are used (Karram and Bhatia, 1990). Another 1-0 polypropylene monofilament suture is secured to the other free end of the graft, and the graft is placed in 0.9% normal saline until needed. Immediate compression is applied to the thigh to constrict perforating vessels. The wound is irrigated and closed in three layers without closing the fascia lata. The area is carefully evaluated for bleeding arteries before closure. Once the thigh closure is complete, a compressive wrap is applied to the thigh and the SCD is replaced (to remain in place for the duration of the hospital stay). The compressive bandage should remain in place for 8 hours postoperatively, and the patient should be encouraged to ambulate early postoperatively (Dwyer and Kreder, 2008). Sterile 0.9% normal saline is injected into the vaginal epithelium, surrounding the urethra to provide hydrodistention and aid in tissue dissection. The authors prefer an inverted-U–shaped incision because it enables adequate exposure to the urethra and bladder and access to the endopelvic fascia and subsequent retropubic space (Fig. 73–2). The top of the incision is approximately 2 cm below the urethral meatus (easily visualized with assistance of an Allis clamp placed immediately below the meatus), and the arms of U should extend to the level of the bladder neck (determined by Foley balloon location). A No. 15 blade knife is used to carry this incision down through the vaginal epithelium, with care to stay above the periurethral and pubocervical fascia (to avoid bleeding and injury to the urethra and bladder). With an Allis clamp and Metzenbaum scissors, thick vaginal epithelial flaps are created. The flaps are retracted with the help of the vaginal ring retractor. Once adequate lateral flaps have been created and the ischiopubic rami are easily palpated, it is appropriate to perforate the endopelvic fascia. It is imperative that the bladder is adequately drained before this maneuver and before later passage of Stamey needles or larger clamps to prevent inadvertent bladder perforation. With the tips of the Metzenbaum scissors pointed upward and to the ipsilateral shoulder, the endopelvic fascia is perforated by remaining directly medial and immediately under the ischiopubic ramus at the superior margin of dissection (Fig. 73–3). Perforation occurs in a superolateral direction, and the dissecting scissors are spread widely to aid in dissection. With the use of blunt finger dissection, the retropubic space is dissected bilaterally (Fig. 73–4). This dissection leads to the connection between the infrapubic and retropubic dissection planes. Simultaneous finger palpation from abdominal and vaginal incisions should be possible, while gently palpating the bladder medially. Aggressive medial mobilization should not be attempted because it may result in bladder injury. Hemostasis should be achieved with bipolar cautery. In the case of women who have undergone prior urethral suspension or sling, more aggressive dissection may be required. The dissection plane into the retropubic space should be immediately adjacent to the periosteum of the pubis, and the dissection should be performed only sharply to minimize risk of injury to the pelvic viscera. Stamey needles or large clamps are passed from above, through the abdominal incision by careful guidance behind the pubis, and passage is in contact with the pubis throughout until brought out lateral to the bladder and through the vagina (Fig. 73–5). Alternatively, large surgical instruments such as Tonsil clamps may also be used (McGuire, 1978; Blaivas and Olsson, 1988). The bladder must be completely drained before passage of the Stamey needles to avoid inadvertent bladder injury. Cystoscopy should be performed with a 70-degree lens after passage of the needles to confirm the integrity of the bladder, by following the course of the needles (while an assistant moves them). Cystoscopy is not practiced consistently by all urologists after needle passage except when there is visible hematuria or another cause for suspicion (Niknejad et al, 2002; Seung-June et al, 2007). It is the authors’ belief that this step eliminates the morbidity and reoperation required for passage of the sling through the bladder and that cystoscopy is an essential step. In the case of a small bladder injury or inadvertent passage of Stamey needles through the bladder, the needles are removed and passed again and the procedure is completed. Extravesical passage is confirmed. One ampule of indigo carmine is given at this point to confirm ureteral efflux during final cystoscopy for tensioning of sling. The Foley catheter is replaced. The ends of graft suture are passed through the Stamey needle eyelets. After marking the center of the graft with a clamp, the Stamey needles are removed and the ends of the suture are brought out through the abdominal incision and tagged with hemostat clamps (Figs. 73-6 and 73-7). The distal aspect of the graft is sutured to the periurethral tissue with two simple 4-0 polyglactin sutures. The vaginal incision is closed with a watertight, running 2-0 polyglactin suture after complete hemostasis is achieved. Before final tensioning of the sling (see Fig. 73-7) the vagina should be closed and the weighted speculum removed to eliminate factors that can affect the final tension. The polydioxanone sutures are tied down above the rectus fascia (see Fig. 73–7) while cystoscopy with a 30-degree lens is performed to visualize adequate coaptation of the proximal urethra. There is approximately a two-fingerbreadth width between the rectus fascia and the sutures once tied down. The amount of tension may vary owing to the mobility of the urethra or desire to create permanent retention in an individual who will have permanent catheterization. The abdominal incision is closed with a subcuticular 4-0 polyglactin suture. The Foley catheter is left to straight drainage, and a vaginal pack moistened with conjugated estrogens is placed. Since McGuire’s reintroduction of the autologous PVS in 1978 with 80% overall success rate, continence rates have been reproducibly satisfactory, even at long-term follow-up (Table 73–3). Earliest series (McGuire, 1987; Blaivas and Jacobs, 1991) with rectus fascial slings include a diverse and complex patient population, with patients having a history of pelvic irradiation, diabetes, spinal cord injury, and pelvic trauma. Poor or no urethral function was associated with 35% of cases (McGuire, 1987). Cure rates among these earliest series were similar (82%). A main cause of failure was urgency and UUI. A majority of the patients who required clean intermittent catheterization postoperatively had a neurogenic bladder and were counseled to expect urinary retention. Harvesting of rectus fascia appears a favored option over fascia lata for autologous material, likely owing to the urologist’s greater comfort with abdominal anatomy. Yet, intermediate and long-term results of fascia lata PVS procedures (Beck et al, 1988; Govier et al, 1997) are comparable to those that use rectus fascia. Beck and colleagues (1988) noted that all three sling failures occurred within the first 22 cases performed and that 10 of the 13 overall failures were due to UUI. Five (3%) of the patients in this study population were extremely bothered by delayed voiding (mean interval of 59.6 days) and underwent a urethrolysis. Govier and associates (1997) administered an independent survey to assess outcomes, yielding worse outcomes than cure rates reported based on their chart review (70% vs. 87.5%, respectively). The vast majority of incontinence reported postoperatively was related to urgency. This patient satisfaction rate was similar to that noted by Brown and associates (2000) with questionnaire-based outcomes. In this group, all of the patients had thigh pain at 1 to 2 weeks and 11% described persistent thigh pain at 6 weeks. Latini and colleagues (2004) used the Crawford fascial stripper to obtain fascia lata. They reported 20% localized numbness at harvest site, 7% with harvest site pain 1 week postoperatively, 5% tendonitis in the harvest site leg, and 1 case of a harvest site hematoma requiring physical therapy. Yet, 83% of respondents indicated that the procedure had a positive effect on their life, 82% would recommend the surgery to a friend, and 83% would undergo the procedure again (Latini et al, 2004). The rectus fascial autologous sling has been effective up to 7 years (Howden et al, 2006) postoperatively with low morbidity when performed by experienced surgeons. Cure rates have ranged from 46% to 97%, although the measurement of outcomes is varied. Patient-derived cure rates are lower than physician-based or chart review–based outcomes. There is no direct correlation between universally accepted objective and subjective measures of improvement or cure for anti-incontinence procedures (Padmanabhan and Nitti, 2006). The most commonly cited reason for failure relates to urgency symptoms. UUI at follow-up is a common reason for patient dissatisfaction. Reported de novo or UUI rates are 2.0% to 20.8% (Mason and Roach, 1996; Zaragoza, 1996; Haab et al, 1997; Chaikin et al, 1998; Cross et al, 1998; Hassouna an Ghoniem, 1999; Morgan et al, 2000; Groutz et al, 2001; Flynn and Yap, 2002; Albo et al, 2007; Mitsui et al, 2007; Onur et al, 2008). Zaragoza and colleagues (1996) report that their three failures had persistent leakage due to UUI (present preoperatively). One of these patients had previous pelvic irradiation and radiation cystitis, and 1 had additional UDS, confirming bladder instability and lack of urethral obstruction. The short-term complication of postoperative retention that occurred in 60% immediately postoperatively is highlighted. Urinary retention persisted in this group for a median of 6.5 days (1 day to 5 weeks) and resolved in 70% within 10 days. Mason and Roach (1996) confirmed the success of a modified rectus fascial sling (smaller graft, 4 × 2 cm) with intermediate follow-up. Twelve (19%) patients had urgency, and 10 (15.9%) had frank UUI. By 6 months these symptoms had resolved in all but 3 (4.8%), who remained on anticholinergic medication. Haab and associates (1997) provided results of long-term follow-up using a self-administered questionnaire to assess success rates and overall patient satisfaction. Among the 3 patients (7.5%) requiring permanent clean intermittent catheterization, 2 had known sacral arc denervation and were expected to have urinary retention. These investigators cited the main reason for failure as urgency symptoms in 62% of patients at follow-up, with 10% reporting de novo urgency. The low reported dry rate (46%) relates to the large preoperative mixed urinary incontinence (MUI) group (67%) as compared with their cure rate for SUI only (73%). Cross and colleagues (1998) advocated the use of a PVS in conjunction with anterior colporrhaphy for repair of cystocele to treat both conditions effectively. Thirty-six patients (63%) had a PVS due to a grade III or IV broad-based cystocele and SUI. Thirty-three (92%) of these cystoceles were cured. These authors reported a 28% postoperative urgency/UUI rate with 19% de novo urgency/UUI. Yet only 3% had persistent UUI requiring treatment; 2.8% underwent further urethrolysis to resolve voiding difficulties. Hassouna and colleagues (1999) reported long-term outcomes of a modified rectus fascial PVS. The modified PVS is a modification of the free rectus fascial strip technique popularized by McGuire that includes fixation of the sling to the pubocervical and periurethral ligaments in a four-quadrant manner (Ghoniem, 1991). After the initial reported 95% success rate by Ghoniem, this study confirms a lower overall rate of 80.8%, when including assessment by a patient quality of life questionnaire. All 15 of the patients with documented failures had UUI or severe irritative symptoms. The additional quality of life assessment highlights the impact of UUI, frequency, nocturia, and pain on patient satisfaction. Chaikin and Blaivas (1998) and Morgan and associates (2000) provide a long-term analysis of the efficacy and quality of life impact of an autologous PVS. Chaikin and Blaivas (1998) reported an SUI cure or improved rate of 92% but also compared instruments for measurement of incontinence (patient assessment, chart review, pad testing, and voiding diary entries). Seventy-five percent of the patients have “complex” SUI, which included “pipe stem urethra,” urethral or vesicovaginal fistula, urethral diverticulum, grade 3 or 4 cystocele, or neurogenic bladder. Concern is raised over the PVS converting an incompetent bladder neck into an obstructing system, with the finding of 23% incidence of DO at 1 year, which increased to 41% at 10 years. The failure rate due to UUI (de novo plus persistent) is similar to that reported by Cross and coworkers (1998) of 22%. Morgan and associates (2000) reported an 85% cure rate at 5 years, yet to achieve this level of cure 14 patients (5.7%) had secondary procedures. This included six collagen injections, three repeat PVS procedures, and five urethrolyses. Eighty percent of these 5 patients had a subsequent return to normal voiding. Chaikin and Blaivas (1998) and Morgan and associates (2000) together demonstrate that when SUI is resolved for more than 1 year after an autologous PVS procedure, the long-term (5 to 10 year) risk of recurrent SUI is low. Groutz and associates (2001) reported outcomes based on whether the PVS was the primary (57%) or secondary anti-incontinence procedure (43%). The latter group had undergone 1 to 3 prior unsuccessful anti-incontinence procedures for recurrent incontinence. The cure rate was significantly higher in patients with primary incontinence than those with recurrent incontinence (74% vs. 59%, P = .006). Yet there were no surgical failures in either group. All of the patients were either cured or improved based on their outcome score, incorporating 24-hour voiding diary, 24-hour pad test, and patient satisfaction. Richter and associates (2001) assessed long-term effects based on a telephone quality of life survey. This study reports high patient satisfaction, whereas 11.8% of the subjects required regular intermittent self-catheterization; 5.8% had to adopt adaptive positions to facilitate voiding. All of the obstructed patients were satisfied due to freedom from urinary incontinence and did not elect urethrolysis. This may relate to an emphasis of preoperative counseling in conveying the possibility of long-term voiding dysfunction after a PVS procedure. Chou and colleagues (2003) provided long-term outcomes for the effectiveness of an autologous PVS in management of MUI. The population included 48.5% with simple SUI and 51.5% with MUI. On univariate analysis of MUI, the higher number of urgency or UUI episodes preoperatively, the more likely it was that the PVS would fail. Yet, women with SUI and concurrent UUI or DO have a successful PVS outcome at a rate comparable to that in women with simple SUI (97% vs. 93%, respectively, P = .33). DO in 26% of those with MUI did not predict a poor outcome. This is contrary to their earlier observations (Chaikin and Blaivas, 1998). The SiSTER trial (Albo et al, 2007), a multicenter, randomized clinical trial, compared the rectus fascial autologous sling to the Burch colposuspension. The two primary outcomes were composite measures of success in terms of overall urinary incontinence measures (no self-reported symptoms of incontinence, increase of less than 15 g in pad weight during a 24-hour pad test, and no medical or surgical treatment for incontinence) and of SUI measures (no self-reported symptoms of SUI and negative stress test), specifically. Whereas success rates were higher for the PVS (47% vs. 38%, P = .01), voiding symptoms were also higher: urinary tract infections (UTIs) (48% vs. 32%), difficulty voiding (14% vs. 2%, P < .001), and postoperative UUI (27% vs. 20%, P = .04). All of the surgical procedures for bladder outlet obstruction (19 of 20) were performed in the sling group. Mitsui and associates (2007) analyzed the risk factors for postoperative voiding difficulty. Patients with a postvoid residual urine volume greater than 100 mL (P = .05) or Petrou and Frank (2001) retrospectively reviewed the safety and efficacy of a repeat PVS for recurrent SUI among 14 women and concluded that repeat PVS is a reasonable treatment option. The original surgeries included autologous PVS (3 patients), cadaveric bone–anchored PVS (3 patients), cadaveric bone–anchored suburethral sling (5 patients), and a vaginal patch sling (3 patients). Postoperative complications included a pelvic abscess related to a prior cadaveric sling and osteomyelitis pubis related to the prior bone anchored sling. One (7%) of the patients developed long-term urinary retention and is on intermittent bladder catheterization; 86% of the patients considered themselves cured or improved. Urethral and bladder neck reconstruction for fistulae and diverticula requires restoration of both anatomy and function. An autologous PVS serves an important role in these reconstructions. Anatomic damage refers to tissue loss and can range from a urethrovaginal fistula to absence of the urethra or vesical neck. The causes of damage include protracted obstetric delivery, anti-incontinence procedures, urethral diverticulectomy or other sequelae of urethral diverticulum, aggressive transurethral resection of the bladder neck, long-term indwelling urethral catheter, pelvic trauma, tumors, and irradiation (Blaivas and Jacobs, 1991). The goals of surgical repair are to restore function and anatomy while fashioning an unobstructed urethra and maintaining continence (Blaivas and Heritz, 1996). Swiertzewski and McGuire (1993) reviewed the records of 14 women who underwent urethral diverticulectomy during a 3-year period. Only 8 (57%) had symptoms of SUI, confirmed on urodynamics in 7. All 7 women were cured with a diverticulectomy and autologous PVS. One patient demonstrated DO. These investigators concluded that the presence of a urethral diverticulum did not compromise the outcomes of the PVS. Chancellor and coworkers (1994) performed a PVS procedure in 14 women with destroyed urethras secondary to long-term indwelling Foley catheter management of neurogenic bladder dysfunction. Greater tension was applied to the sling suspension to achieve urethral closure. Ten patients had simultaneous intestinal augmentations or diversions, 2 had concurrent suprapubic tube placement, and 2 had adequate bladder capacity and compliance and only had PVS performed. All of the patients achieved continence. The PVS is a simpler addition and avoids the risk of fistula formation associated with bladder neck closure, which has historically been used in this population. Blaivas and Heritz (1996) performed a retrospective study of 49 women who underwent a one-stage urethral reconstruction at up to 11-year follow-up. Forty-one of these women had a concomitant PVS placed for management of preoperative SUI. Only 1 patient experienced obstruction and DO after PVS placement, requiring incision and subsequently is continent. Three of 5 women who had Pereyra repairs for SUI required secondary PVS and are also now continent. Faerber (1998) reported on 16 women who had simultaneous PVS and diverticulum repairs after UDS. All were either significantly improved (12%) or cured (88%), and only 2 patients developed de novo urgency. The average time for achieving adequate bladder emptying after repair was 5 weeks. For the initial 2 weeks, a Foley catheter is usually in place for urethral healing. Rovner and Wein (2003) reported on the circumferential urethral diverticulum repair among 9 patients who received either end-to-end urethroplasties or dorsal urethroplasties. Based on preoperative SUI status or evidence of open bladder neck on preoperative cystography, 8 patients were recommended to have a concurrent PVS procedure performed. All patients had a rectus fascial PVS sling placed, except 1 who requested a porcine PVS and 1 who refused to have PVS repair. All patients were continent postoperatively, except the patient who refused the PVS procedure and developed de novo SUI. Flisser and Blaivas (2003) evaluated the results of 74 women with urethral pathologic processes that required vaginal flap reconstructions. A majority of the women had required reconstruction for a diverticulum or urethral fistula secondary to iatrogenic causes. Fifty-six of these women had a concomitant PVS placed. Eighty-seven percent (54 women) considered themselves cured postoperatively. Three of 4 patients who had persistent SUI had failed a modified Pereyra procedure but were cured at reoperation. One patient was continent after operative revision of the PVS for obstruction and significant UUI. The autologous fascial PVS remains a gold standard with efficacious and durable outcomes, but, owing to the morbidity of graft procurement, cadaveric allograft slings were introduced. This was to provide decreased morbidity, reduce operative time, and minimize pain (Table 73–4). There are limited outcome data owing to questions surrounding efficacy and durability. Early literature used cadaveric frozen or freeze-dried fascia lata with a variety of fixation methods, including suture fixation and bone anchors (Handa et al, 1996; Wright et al, 1998; FitzGerald et al, 1999; Brown and Govier, 2000; Carbone et al, 2001; Flynn and Yap, 2002; O’Reilly and Govier, 2002; Walsh et al, 2002; Amundsen et al, 2003; Richter et al, 2003; Almeida et al, 2004; Howden et al, 2006). Experience has shown that tissue processing techniques can have deleterious effects on cadaveric sling outcome (Nazemi et al, 2008). Lemer and associates (1999) found differences in both tissue integrity and durability between freeze-dried fascia and solvent-dehydrated fascia. They theorized that ice crystal formation characteristics of tissue freezing may disrupt the collagen matrices, yielding poor strength properties. Failure rates for frozen or freeze-dried grafts range from 6.0% to 37.6% (Handa et al, 1996; Wright et al, 1998; FitzGerald et al, 1999; Brown and Govier, 2000; Carbone et al, 2001; Flynn and Yap, 2002; O’Reilly and Govier, 2002; Walsh et al, 2002; Amundsen et al, 2003; Richter et al, 2003; Almeida et al, 2004; Howden et al, 2006). Handa and associates (1996) reported on some of the earliest short-term outcomes for cadaveric fascia lata, with promising cure rates. Two of their patients (12%) developed abdominal wound infections, which resolved with local care, including drainage. They reported de novo urgency in 36% of the cases, whereas DO resolved in one of four cases. Two of the 3 patients who had SUI recurrence were continent after a synthetic midurethral sling. FitzGerald and associates (1999) reported on 35 patients undergoing cadaveric fascia lata sling placement with a high failure rate of 17%. Symptom recurrence was seen very early (1 week to 5 months). Histopathologic analyses of the retrieved material indicated ongoing processes in the failed graft: disorganized remodeling, areas of graft degeneration, and evidence of immune reaction. These findings led them to conclude that freeze-dried, irradiated donor fascia lata grafts should not be used for urogynecologic procedures due to the high material failure rate. Three studies (Amundsen et al, 2000b; Walsh et al; 2002; Richter et al, 2003) documented good results without significant adverse outcomes using freeze-dried fascia lata. Amundsen and associates (2000b) utilized questionnaires and pad testing to assess incontinence outcomes in a complex population (80% having had prior pelvic surgery and 49% having had at least 1 prior anti-incontinence procedure). Sixty-five percent of the group had MUI preoperatively, with 41% of them having resolution of UUI; 15% developed de novo UUI, and no erosions were present. In 1 patient who underwent reoperation for a failed sling the fascia lata was found completely intact. The sling was torn from its nonabsorbable suture material, similar to a previously reported case (Chaikin and Blaivas, 1998; Amundsen et al, 2000b). Walsh and colleagues (2002) prospectively evaluated 31 women with promising short-term follow-up. There was complete resolution of SUI in 94% at both 4 months and 1 year. There was also a reduction in the incidence and severity of urgency and UUI after surgery, reflected in the declining use of anticholinergic surgery from before to 4 months and 1 year postoperatively (55% to 32% to 26%). Richter and colleagues (2003) conducted a prospective long-term study using validated questionnaires for a follow-up of 48 months. Difficulty emptying the bladder was described by 58.2% of patients at 12 month follow-up, with 34.2% describing it as slight. By 48 months, 50% of patients continue to have difficulty emptying their bladders. Patients reported a 90.2% satisfaction rate at 12-month follow-up and continued to be satisfied at 48-month follow-up (85.7%). Two groups documented high failure rates with freeze-dried allograft (Carbone et al, 2001; O’Reilly and Govier, 2002). Carbone and associates (2001) used cadaveric fascia with titanium bone anchors for placement bilaterally in pubic bones. They reported a 38% failure rate and 17% reoperation rate at short-term follow-up of 11 months. Average time to reoperation was 9 months (3 to 15 months). Intraoperative findings at reoperation revealed the titanium anchors to be in position, the polypropylene sutures to be intact, and retropubic fibrosis and scarring of the urethropelvic ligament suggesting appropriate placement of the sling. All of the allogenic fascia appeared to be fragmented, attenuated, or simply absent. These authors have subsequently abandoned the use of cadaveric fascia allografts in all the PVS procedures at their institution. O’Reilly and Govier (2002) reported high intermediate failure rates in 121 women. Eight patients had recurrent SUI at a mean of 6.5 months (4 to 13 months); 7 of the 8 women had previous incontinence surgery and had multiple comorbidities, including neurologic disease, diabetes, previous pelvic irradiation, and previous pelvic surgery. Based on these results and findings by Lemer and associates (1999), this group discontinued use of fresh frozen grafts and switched to solvent dehydrated cadaveric fascia and dermal grafts. Some studies support the efficacy of solvent-dehydrated fascial slings (Frederick and Leach, 2005; Onur and Singla, 2005; Pianezza et al, 2007; Nazemi et al, 2008), yet previous reported failures coupled with the consistent success and rapid adoption of synthetic midurethral slings has led to abandonment of all types of cadaveric allograft at most centers. Thus there are few data to assess long-term outcomes and define sling outcomes after solvent dehydration techniques (Nazemi et al, 2008). Huang and colleagues (2001) were the earliest to report unfavorable experiences using solvent-dehydrated allograft in 18 women at short-term follow-up. They reported a 27.8% failure rate with full recurrence of incontinence and subsequently stopped using all allograft as a sling material. On reoperation with an autologous PVS for recurrent SUI the allograft remained only rudimentary and was very friable. Histologic examination of the retrieved allograft revealed wavy collagen fibers with loosely packed fibroblasts and focal areas of degeneration. Carey and Leach (2004) prospectively compared octogenarians and younger women undergoing treatment for incontinence with bone-anchored solvent-dehydrated cadaveric fascia lata. No statistically significant difference in outcome parameters was identified. The rates of de novo urgency were similar between the two groups, yet persistent urgency was greater among the octogenarians. Onur and associates (2005) reported 80% cure rates at 12-month follow-up. In 5 patients (20%) complete recrudescence of symptoms occurred within 6 months of surgery. Frederick and associates (2005) reported on prospective, intermediate-term results for cadaveric prolapse repair with bone-anchored sling (CaPS), with dry/cure rate and improvement rate of 56% and 82%, respectively. There was one case of osteitis pubis related to use of transvaginal bone anchor fixation that resolved with conservative management. Fifty-six percent of the failures occurred after 12 months of follow-up: 80% of these women were satisfied and 77% stated that they would undergo the CaPS procedure again. Using validated questionnaires (i.e., Urogenital Distress Inventory, Short Form [UDI-6]; Incontinent Impact Questionnaire [IIQ-7]), Pianezza and associates (2007) reported on similar levels of long-term patient satisfaction. Patients with preoperative MUI were at the greatest risk for postoperative dissatisfaction. Nazemi and associates (2008) described durable improvements in incontinence episodes, patient satisfaction, and validated quality of life end points when comparing a CaPS and CaTS (cadaveric sling placement alone) cohort. Yet there was a reduction in dry rates with extended follow-up (24 months, 48 months, and 60 months), especially in the CaTS group (23%, 18%, and 9% respectively); 4% of patients presented with sling extrusion and 4% required sling incision and urethrolysis. Because of early and intermediate graft failure with cadaveric fascia lata, surgeons were prompted to use alternative materials: cadaveric dermal grafts. Crivellaro and associates (2004) presented their prospective series of patients treated with bone-anchored Repliform cadaveric human dermal allograft (LifeCell Corp., The Woodlands, TX) PVS. There was a 22% failure rate, yet based on patient report there was average incontinence improvement at 9 months of 85% and at 18 months of 80%. The 2 patients requiring long-term intermittent catheterization had neurogenic bladders. Owens and Winters (2004) assessed outcome and patient satisfaction with Duraderm allograft (C.R. Bard, Covington, GA). Initial results were promising, with a dry rate of 68% and improved rate of 24%. At intermediate follow-up only 32% of the patients were dry and 36% noted improvement. Two of the 8 patients who experienced graft failure underwent periurethral injections with significant improvement, and 1 is dry after an autologous sling procedure. Surgical reexploration revealed almost complete absence of graft material, without evidence of infection or excessive inflammatory response. Six studies have compared the outcomes of women undergoing a PVS procedure using either autologous or cadaveric allograft fascia (Wright et al, 1998; Brown and Govier, 2000; Flynn and Yap, 2002; Almeida et al, 2004; Howden et al, 2006; Onur and Singla, 2008). Four groups found the outcomes comparable with equally high success rates and no negligible difference in complications. They concluded that allograft fascia lata may be used as an alternative to autologous fascia for PVS, to reduce operative time and decrease postoperative pain and disability (Wright et al, 1998; Brown and Govier, 2000, Flynn and Yap, 2002; Onur and Singla, 2008). With long-term follow-up, two groups noted superior continence outcomes in the autologous group (Almeida et al, 2004; Howden et al, 2006). Almeida and associates (2004) did not report adverse outcomes in either group. Howden and colleagues (2006) found recurrent symptoms occurred in a higher proportion in the cadaveric group (39.6%) compared with the autologous group (28.3%; P = .04). The reoperation rate was also higher for the allograft group (12.7% vs. 3.3%, P = .003). A number of different sling materials are available and have been discussed. Because of the morbidity of autologous fascial harvest, high failure rates of allograft materials, and high erosion rate with synthetic PVS materials, xenografts are an attractive option. They are associated with a low rate of infection, rejection, or extrusion, owing to their incorporation into host tissue (Rutner et al, 2003). Studies present in the literature utilize porcine dermis (Pelvicol), porcine SIS, and bovine dermis as xenografts for use in PVS surgery (Arunkalaivanan and Barrington, 2003; Rutner et al, 2003; Abdel-Fattah et al, 2004;Wiedemann and Otto, 2004; Giri et al, 2006; Wilson et al, 2008) (Table 73–5). Rutner and colleagues (2003) were the first to describe the use of porcine SIS as a bone-anchored PVS in 152 patients with long-term follow-up. They reported cure rates comparable to those with the autologous sling. Among the 7 patients who experienced graft failure (4.6%), 5 had recurrent SUI within 3 months of surgery and the other 2 had recurrences at 9 and 11 months postoperatively. Two of the failures were related to the bone anchors. One patient had repeat porcine SIS PVS, with continued failure to relieve incontinence and 1 patient became dry after carbon bead bulking injections. One patient achieved continence after a urethrolysis 2 years postoperatively. At reoperation, grossly no evidence of the implanted SIS material was found. Biopsy specimens of the periurethral tissue revealed fibrous and muscular tissues with a few remnants of SIS. Wiedemann and Otto (2004) performed the first histopathologic examination of porcine SIS in a series of 15 women with SIS PVS. Three reoperations (20%) were necessary owing to recurrent SUI at a mean of 12.7 months. All 3 patients underwent reoperation with implantation of a polypropylene mesh and achieved immediate continence. Biopsy specimens from the SIS band under the vaginal mucosa revealed only focal residues of SIS without any evidence of tissue reaction. There was also no evidence of a significant immunologic or chronic inflammatory reaction. These authors concluded that the advanced incorporation of the implant would lend to good biocompatibility. Arunkalaivanan and Barrington (2003) first reported on Pelvicol in a randomized, short-term trial comparing Pelvicol PVS with the tension-free vaginal tape (TVT) procedure. Questionnaire-based cure rates were comparable between the two: 85% in the TVT group and 89% in the Pelvicol implant group. Abdel-Fattah and associates (2004) presented the 3-year follow-up data for this prospective trial. Cure rates remained high and comparable between the two groups: 79.1% for the TVT group and 77.8% for the Pelvicol group. There was no statistical difference in regard to complication rates or postoperative pad score. Giri and colleagues (2006) compared the 3-year efficacy of Pelvicol with autologous rectus fascia in 101 consecutive, nonrandomized patients. Although porcine dermis reduced the associated surgical morbidity, there were significantly inferior long-term cure rates as compared with the autologous PVS. Treatment failure occurred by 9 months in the autologous group and by 24 months after the Pelvicol sling. Repeat UDS indicated SUI as the cause of treatment failure in 18 of 20 (90%) women treated with porcine dermis but only in 3 of 8 (6.5%) women after a rectus fascial sling. These authors concluded that Pelvicol should not be used as a substitute for rectus fascia. Bovine dermis is the most recently reported material used for a xenograft PVS (Wilson et al, 2008). Women at high risk for sling failure (with advanced age, previous surgical failure, intrinsic sphincter deficiency) underwent either a bovine dermis or autologous rectus fascia PVS procedure with short-term follow-up. Global cure rates and SUI cure rates were not statistically different between the two groups. Four women (8.3%) in the autologous group were reoperated with injections, repeat autologous PVS placement, and anterior colporrhaphy with interposition graft. Two women (5.4%) in the bovine dermis group underwent additional interventions: injections and a repeat autologous PVS placement. Biopsy specimens of the bovine dermis sling material during reoperation (for SUI recurrence at 3 months) revealed the sling was replaced by fibrosis, hemorrhage, and mild chronic inflammatory infiltrate, with no acellular component. Tissue breakdown, represented by intermittent areas of myxoid degeneration, was present and may indicate evidence of early graft failure. Key Points: Pubovaginal Sling Outcomes and Urethral Reconstruction The incidence of sling erosion is dependent on the composition of sling material. Synthetic slings erode 15 times more often into the urethra and extrude 14 times more often into the vagina than autologous, allograft and xenograft slings (Blaivas and Sandhu, 2004). This is based on a meta-analysis of peer-reviewed literature in 1997, citing a urethral erosion incidence of 0.02% and vaginal extrusion of 0.007% in 1515 patients who underwent synthetic slings. This is compared with a urethral erosion incidence of 0.003% and vaginal extrusion of 0.0001% in 1715 patients undergoing autologous and allograft slings (Leach et al, 1997). In subsequent studies, most erosions have been associated with synthetic slings, particularly woven polyester slings (Summit et al, 1992; Bent et al, 1993; Weinberger and Ostergard, 1995; Myers and LaSala, 1998
Pubovaginal Sling
Assessment of Intrinsic Sphincter Deficiency
Anatomy and Mechanics
Sling Materials
Autologous Materials
Allograft Materials
Xenograft Materials
Synthetic Materials
TYPE
DESCRIPTION
BRANDS
I
Pores >75 µm; macroporous
Atrium, Trelex, Marlex, Prolene
II
Pores <10 µm; microporous
PTFE: GORE-TEX, Surgical Membrane, Dualmesh
III
Macroporous with multifilamentous or microporous components
PTFE: Teflon, braided Dacron mesh (Mersilene), braided polypropylene mesh (Surgipro), perforated PTFE patch (MicroMesh)
IV
Submicronic pore size
Silastic, Cellcard (polypropylene sheeting)
TRADE NAME
COMPOSITION
DETAILS
Mersilene
Polyethylene terephthalatae
Multifilament fibers
Very porous, becomes firmly embedded in native tissues
Teflon
Polytetrafluoroethylene (PTFE)
Multifilament
GORE-TEX
Expanded PTFE
Very flexible
Silastic
Silicone plus woven polyethylene terephthalate
Minimal tissue reaction, which facilitates removal or revision if necessary
ProteGen
Synthetic mesh impregnated with collagen matrix
Removed from market secondary to high rate of vaginal extrusion
Marlex, Prolene
Polypropylene
Monofilament with open-weave pattern
Operative Procedure
Preoperative and Preliminary Steps
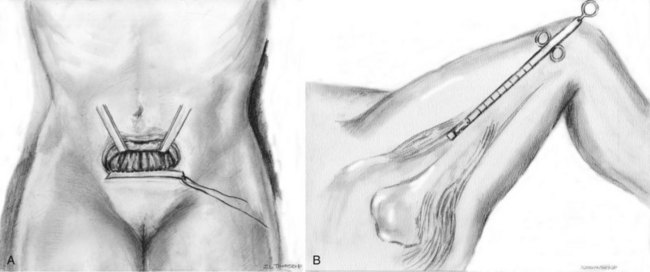
Graft Harvest
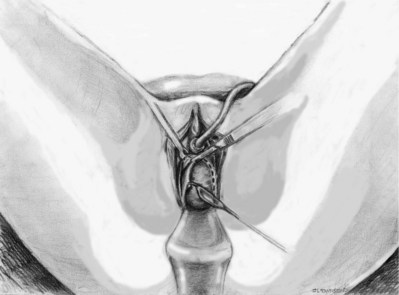
Vaginal Approach
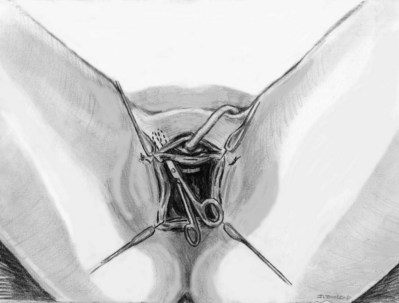
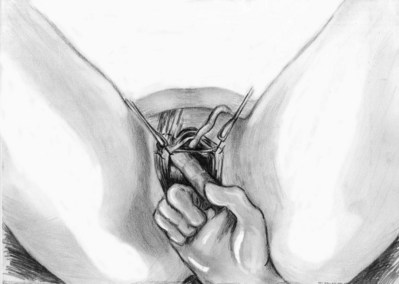
Sling Placement and Fixation
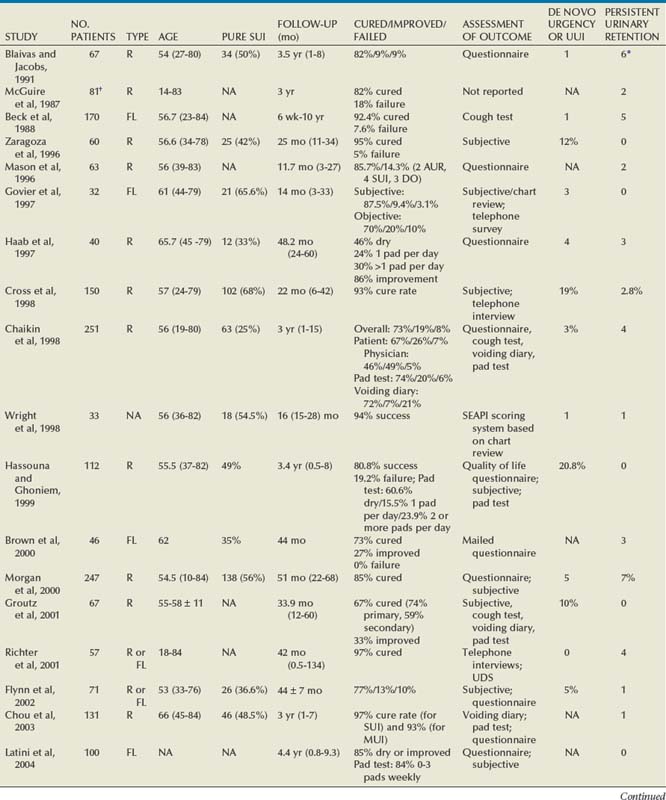
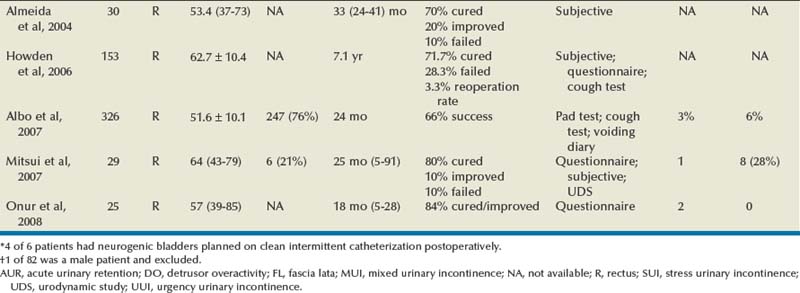
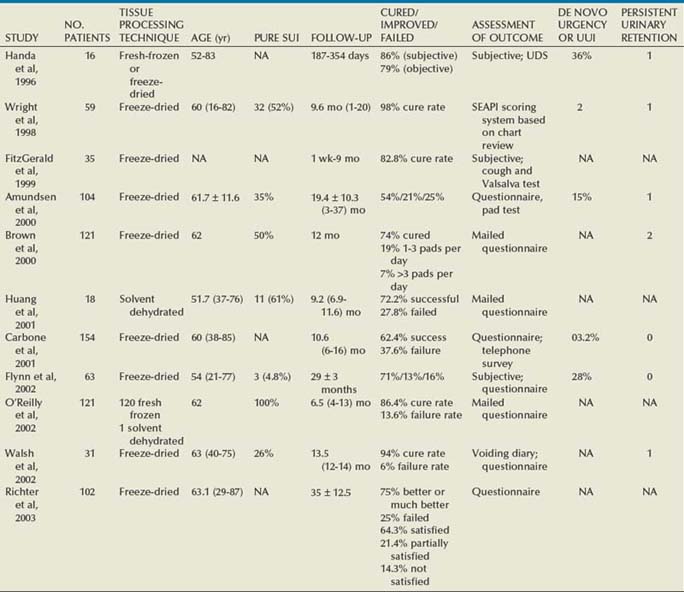
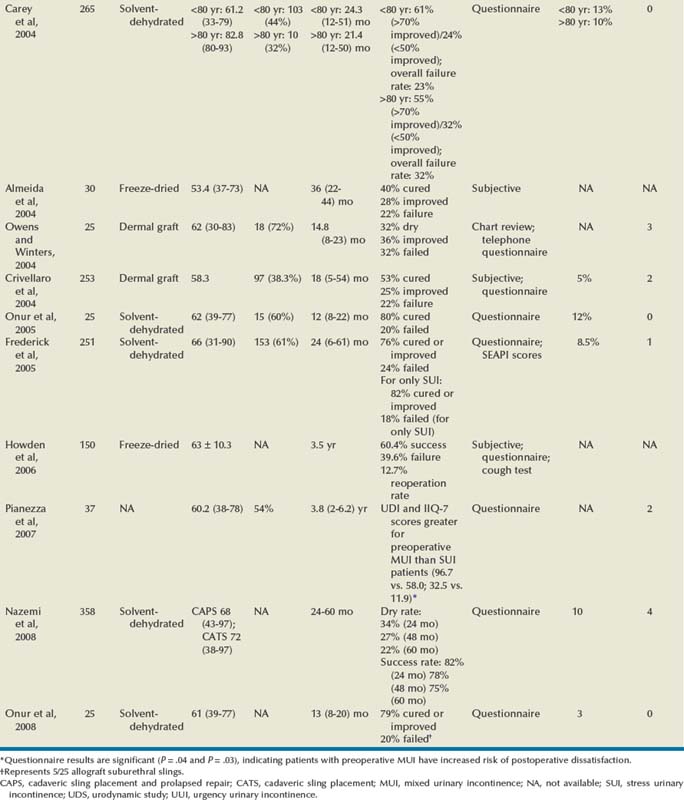
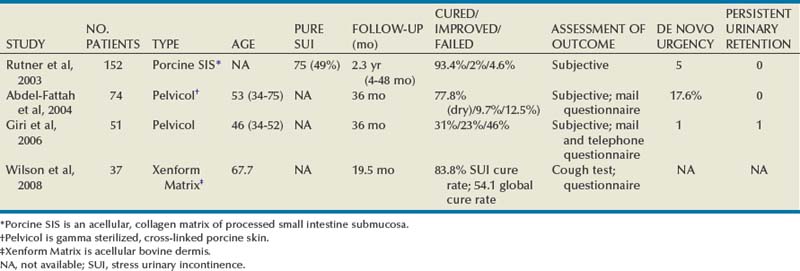
Outcomes
Autologous Pubovaginal Sling
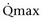 of less than or equal to 20 mL/s (P = .09) in preoperative urodynamics were more likely to require prolonged intermittent self-catheterization. In this group, 28% needed prolonged intermittent self-catheterization (range 4 to 40 months). There have been heterogeneous outcomes in regard to risk factors for postoperative voiding difficulty. This may relate to differences in surgeon skill or surgical method. Although postoperative urgency and UUI are strongly related to failure, there are no preoperative risk factors that consistently predict outcomes after a PVS operation (Mitsui et al, 2007).
of less than or equal to 20 mL/s (P = .09) in preoperative urodynamics were more likely to require prolonged intermittent self-catheterization. In this group, 28% needed prolonged intermittent self-catheterization (range 4 to 40 months). There have been heterogeneous outcomes in regard to risk factors for postoperative voiding difficulty. This may relate to differences in surgeon skill or surgical method. Although postoperative urgency and UUI are strongly related to failure, there are no preoperative risk factors that consistently predict outcomes after a PVS operation (Mitsui et al, 2007).
Value of Autologous Pubovaginal Sling in Urethral Reconstruction
Allograft
Xenograft
Complications
Erosion and Extrusion
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Slings: Autologous, Biologic, Synthetic, and Midurethral
• The autologous PVS is associated with cure rates of 46% to 97% with variable measurements of outcome utilized.