General Approaches
- Abrupt onset of hypertension.
- Blood pressure ≥160/100 mm Hg.
- Considerable target-organ damage.
- Positive result of a highly specific diagnostic test.
- Adequate response to therapy for the specific type of secondary hypertension.
More than 95% of Americans with hypertension have no specific cause for their elevated blood pressure (BP). It is important, however, to consider the possibility that newly diagnosed hypertension has a specific cause, for three reasons. First, BP control is often difficult to achieve in people with secondary causes of hypertension; diagnosing it early is likely to get BP to goal more quickly. Second, and particularly important in younger people, diagnosing and treating secondary hypertension will reduce the future burden of treatment (both expenditures for pills and follow-up and adverse effects of therapy) and improve the quality of life. For some secondary causes, specific and potentially curative therapy is available. Lastly, routine consideration of secondary causes when the diagnosis of hypertension is first made will ensure that at least once during the person’s lifetime the possibility of secondary hypertension will be entertained. The risks and benefits of further testing can therefore be critically evaluated.
Patients with an identifiable secondary cause of hypertension typically present with a relatively abrupt onset of symptoms (BP ≥160/100 mm Hg) and with considerable target-organ damage. They typically do not respond as well to lowering BP and to antihypertensive drug therapy as do patients with primary hypertension. The BP-lowering response to specific antihypertensive drugs may offer important clues to the presence and type of secondary hypertension; for example, patients with early renovascular hypertension often have an impressive BP-lowering response to an angiotensin-converting enzyme (ACE) inhibitor and those with bilateral adrenal hyperplasia as a cause of primary aldosteronism respond well to spironolactone, but not vice versa. The most common forms of secondary hypertension and useful tests for each will be discussed individually. The choice of tests and the order in which they are obtained depend not only on the pretest probability of the disease, but also on safety, availability, local expertise with the test, and its cost.
Renovascular Hypertension
- Renovascular hypertension (RVHT) is the most common cause of secondary hypertension in the United States.
- RVHT is an elevation of blood pressure (BP) due to activation of the renin–angiotensin system in the setting of renal artery occlusive disease.
- The diagnosis of RVHT can be made only if BP improves following intervention, thereby making RVHT a retrospective diagnosis.
- The presence of anatomic renal artery stenosis (RAS) is not synonymous with RVHT.
- Progressive and occlusive renovascular disease may lead to impaired kidney function, termed “ischemic nephropathy.”
Recognition of important clinical clues for RVHT is paramount in the clinical diagnosis of this condition. RVHT probably occurs in less than 1% of unscreened patients with mild hypertension. By comparison, 10–30% of white patients with severe or refractory hypertension may have renal artery disease. Pertinent clinical clues for RVHT are summarized in Table 42–1.
Severe or refractory hypertension |
Age of onset younger than 30 years or older than 55 years |
Abrupt acceleration of stable hypertension |
Severe hypertension in the setting of generalized atherosclerosis |
Systolic–diastolic bruit in the epigastrium |
Flash pulmonary edema |
Unexplained azotemia |
ACE inhibitor- or ARB-induced renal dysfunction |
Essential or primary hypertension is the most common form of Hypertension, occurring in >90% of the more than 50 million Americans with elevated BP (Table 42–2). Of the 5–10% of hypertensive patients with secondary hypertension, RVHT accounts for 0.2–3%. However, at autopsy the prevalence of anatomic RAS attributable to atherosclerosis (ASO) in the elderly is quite common. Clinically, RAS may coexist in 20–25% of patients undergoing cardiac catheterization for coronary artery disease (CAD). Similarly, approximately 6% of patients with end-stage renal disease (ESRD) have a concomitant diagnosis of RAS. However, it is unclear whether the occlusive RAS was etiologic in the development of end-stage kidney failure.
Classification | Prevalence (%) |
|---|---|
Essential (primary) hypertension | 90 to 95 |
Secondary hypertension | 5 to 10 |
Renal | 2.6 to 6.0 |
Renovascular hypertension | 0.2 to 3.0 |
Endocrine (primary aldosteronis, pheochromocytoma, thyroid disease, etc.) | 1 to 2 |
The etiology of RAS is usually attributable to ASO or fibromuscular disease (FMD). As can be seen in Table 42–2, ASO accounts for over 70% of RAS. It is generally seen in an older hypertensive population with concomitant diffuse ASO in other vascular beds (eg, coronary, carotids, and peripheral circulation). The RAS lesion due to ASO occurs at the ostium or in the proximal 2 cm of the renal artery. In contrast, FMD accounts for 20–25% of RAS and is typically seen in younger female hypertensive patients. Table 42–3 lists other less common causes of RAS.
Cause | Prevalence (%) | Clues/characteristics |
|---|---|---|
Atherosclerosis (ASO) | 70 | Older patients (>55 years of age) Concomitant diffuse ASO in other vascular beds Ostial or proximal (2 cm) renal artery lesions |
Fibromuscular dysplasia (FMD) | 20 | Younger women Unclear etiology |
Others | Extrinsic compression by tumor Retroperitoneal mass Arterial dissection Vasculitis Aneurysm |
The pathophysiology of renovascular hypertension is best explained by the sentinel animal experiments by Goldblatt. These animal models consist of occluding one or both renal arteries with constricting clips. The two-kidney one-clip (2K-1C) model represents unilateral RAS whereas the two-kidney two-clip (2K-2C) model represents bilateral RAS. Unilateral stenosis in a solitary functioning kidney, such as in renal transplant patients, is represented by the one-kidney one-clip (1K-1C) model. Both the 2K-2C and 1K-1C models share similar features.
The mechanism of development of hypertension is mediated via the renin–angiotensin–aldosterone system (RAAS) with salt and water retention. In unilateral RAS (2K-1C model), renal perfusion pressure is decreased in the kidney distal to the stenosis, which leads to increased renin production, which in turn forms angiotensin II (AT II). AT II causes vasoconstriction directly and also stimulates aldosterone production, which causes salt and water retention. The normal contralateral kidney undergoes a pressure natriuresis, which maintains volume status. Due to the constantly elevated levels of renin in the 2K-1C model, this form of RAS is referred to as renin-mediated hypertension.
On the other hand, in the 2K-2C or 1K-1C models representing bilateral RAS or RAS to a solitary kidney, there is an initial increase in renin, which in turn causes an increase in AT II and aldosterone. As in the model described, resultant salt and water retention occurs, but the absence of a normal contralateral kidney prevents pressure natriuresis. Suppression of renin occurs due to volume expansion attributed to the increases in salt and water retention. This form of hypertension is considered volume mediated, whereas the 2K-1C model of unilateral RAS is renin mediated.
Stenosis that causes hemodynamic changes with a reduction in renal perfusion pressure is called critical stenosis. In Goldblatt’s experimental models, 80–85% renal artery constriction induces significant hemodynamic changes. In humans, a >75% degree of RAS is thought to cause critical hemodynamic changes. Clinically, this is of importance as modest RAS may be present in many older patients, but may not be sufficient to produce hemodynamically significant lesions.
There is an immediate rise in BP as a result of increased levels of renin. This initial rise in BP, whether in the 2K-1C or in the 1K-1C model, is renin–angiotensin mediated. Removal of the stenosis reverses the hypertension.
This phase lasts for few days to many weeks in the experimental model. Salt and water retention occur, along with a subsequent fall in plasma renin. Nevertheless, in this phase, removal of the stenosis may reverse the hypertension.
Over time, vascular changes and renal parenchymal disease may develop due to the hemodynamic and nonhemodynamic effects of AT II. The importance of this phase is that removal of the stenotic lesion fails to correct the hypertension. In this phase, blockade of RAAS may not decrease the BP.
The two major goals of the evaluation of the hypertensive patient are to recognize clinical clues for secondary forms of hypertension and to identify evidence of target-organ damage (TOD) from the hypertension. The clinical clues suggestive of RVHT are listed in Table 42–1
RVHT should be suspected in patients presenting with severe, sudden-onset hypertension prior to 30 years of age or after 55 years of age. For reasons that are not well understood, RVHT is relatively less common in African-Americans in whom severe hypertension is most frequently essential. Because fibromuscular dysplasia occurs in younger patients (mainly women), those presenting with hypertension before 30 years of age should be suspected of having RVHT. However, RVHT due to ASO is likely to present in older patients with significant hypertension in the setting of generalized ASO in other vascular beds. Malignant hypertension with neurologic symptoms and advanced fundoscopic changes with papilledema should raise the possibility of RVHT. Similarly, severe or refractory hypertension defined as hypertension requiring three or more drugs as well as severe hypertension with heart failure/flash pulmonary edema may also be one of the presenting features of RVHT. Importantly, this sudden onset of worsening azotemia after the institution of an angiotensin-converting enzyme (ACE) inhibitor or AT II receptor blockers (ARB) should suggest RVHT, especially bilateral RAS or RAS with a solitary functioning kidney. In such patients, with all of their functioning kidney mass distal to a critical RAS, maintenance of the glomerular filtration rate (GFR) is dependent upon efferent arteriolar vasoconstriction mediated by AT II. Once this preservation of function is lost after administration of an ACE or ARB, a decline in renal function is seen.
The presence of severe stage II hypertension (greater than 160–100 mm Hg) may be a critical clue to the presence of RVHT. The presence of an abdominal bruit in the setting of increased BP is also a strong clinical clue to RAS. The bruit is systolic–diastolic in nature and is located near the epigastrium. This is seen more commonly in fibromuscular dysplasia and, in fact, correlates with surgical outcomes. The absence of such a bruit does not exclude RAS. The presence of stage III or IV hypertensive retinopathy on fundus examination is highly suggestive of RVHT. Evidence of diffuse ASO in the peripheral vascular, coronary, and cerebral vascular beds may be suggestive of RVHT due to ASO renal artery disease in the older hypertensive population.
By definition RVHT requires an elevation of BP due to the activation of the renin–angiotensin system in the setting of renal artery occlusive disease. The diagnosis of RVHT can be made only if BP improves after a correction of RAS, thereby making RVHT a retrospective diagnosis. The primary goal in screening for RVHT is to identify a subset of patients who may have a reversible hemodynamic cause of their hypertension and/or renal dysfunction. Table 42–4 summarizes the specificity and sensitivity of these diagnostic modalities
Test | Sensitivity (%) | Specificity (%) |
|---|---|---|
Plasma renin activity (PRA) | 50–80 | 84 |
Functional studies | ||
Captopril PRA | 74 | 89 |
Captopril scintigraphy/renography | 85–90 | 93–98 |
Anatomic studies | ||
Duplex ultrasound scanning | 90 | 90–95 |
Spiral (helical) computed tomography scanning | 98 | 94 |
Magnetic resonance Angiography | 90–100 | 76–94 |
Hypokalemia may be a surrogate marker of hemodynamically significant renal artery occlusive disease secondary to stimulation of the renin–angiotensin system with secondary hyperaldosteronism. The hyperaldosteronism results in urinary sodium retention and kaliuresis, which may be responsible for the development of hypokalemia.
RVHT may be seen in patients with or without renal dysfunction. RVHT and possible ischemic nephropathy may be suspected in patients with unexplained azotemia occurring in the setting of generalized ASO and asymmetric kidney sizes (possibly due to the occlusive RAS). As noted previously, azotemia following the administration of an ACE inhibitor or ARB is a strong clinical clue suggestive of hemodynamically significant renal artery disease.
Historically, plasma renin activity (PRA) was measured to evaluate patients with RVHT. PRA was determined indirectly by measurement of AT I because the amount of AT I produced from angiotensinogen is proportional to the renin enzyme concentration. This reaction is dependent on the amount of angiotensinogen present and can underestimate the renin concentration in patients with severe heart or liver failure who have markedly low levels of angiotensinogen. Direct renin measurements are now determined by specific monoclonal antibodies to renin. Direct renin assays are now available clinically and may offer clinical advantages in terms of accuracy and more rapid reporting of results (a prior PRA level of 1 ng/mL/hour converts to a direct renin level of 8.4 mU/L).
Unfortunately, PRA has been an insensitive method of screening with elevated levels present in only 50–80% of patients with RVHT. Moreover, up to 15% of patients with essential hypertension have elevated PRA levels, making PRA a nonspecific determinant of RVHT. Although infrequently used in usual medical practices today due to its low sensitivity and specificity, a very low PRA (if plasma renin activity is very low, <1 ng/mL/hour, in the absence of drugs known to suppress renin) can strongly argue against RVHT as the cause of elevated BP. Captopril-stimulated PRA testing may be preferable to PRA determination alone in the evaluation of the patient with RVHT.
Commonly used radionuclide agents include technetium-99m diethylenetriaminepentaacetic acid (DTPA), which is used as a marker of GFR because it is exclusively excreted by glomerular filtration, and technetium-99m-labeled mercaptoacetyltriglycine (MAG3), which is used to approximate the renal plasma flow rate and, in contrast to DTPA, is excreted both by glomerular filtration and tubular secretion.
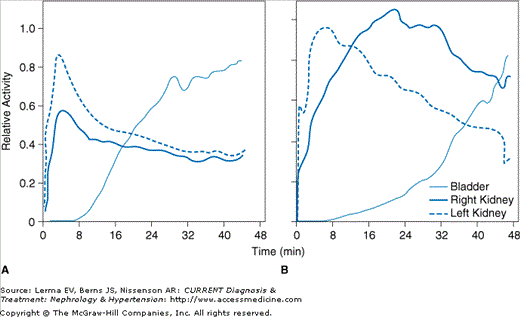
Since the ischemic kidney is dependent upon the effects of AT II to induce efferent artery vasoconstriction to maintain the GFR, the introduction of captopril is expected to lower the GFR of the affected kidney distal to the stenosis. The results after captopril are demonstrated by a decreased uptake of DTPA (decreased GFR) with little change in MAG3 uptake (preserved renal plasma flow rate), but a delayed excretion phase of MAG3 compared to renal scans without captopril provocation (Figure 42–1). Limitations of this technique for screening include decreased sensitivity in patients with renal dysfunction. A positive finding provides clear evidence that the occlusive disease is hemodynamically significant and that intervention is likely to improve BP control.
Figure 42–1.
Captopril scintigraphy/renography. A Tc-DTPA time–activity curve at baseline (A) and after captopril (B) in a patient with stenosis of the right renal artery. The diagnosis of renal artery stenosis is based on asymmetry of renal size and function, as well as a delayed time to maximal activity (>11 minutes), significant asymmetry of the peak activity of each kidney, marked cortical retention of the radionuclide, and marked reduction in calculated glomerular filtration rate of the ipsilateral kidney. (Adapted with permission from Nally JV et al: Advances in noninvasive screening for renovascular hypertension disease. Cleve Clin J Med1994;61:328.)
Atherosclerotic lesions are ostial or proximal within the first 2 cm of the renal artery, and typically do not occur in the distal portion of the renal arteries.
Medial fibroplasia will appear as a “string of beads”(the beads are of larger caliber than the artery), and is often located at the mid to distal portion of the renal artery.
The gold standard in detecting renal artery occlusive disease remains renal angiography/arteriography because it provides maximum information about the vascular architecture as well as an opportunity for intervention if hemodynamically significant lesions are found. With the advent of digital subtraction angiography, less contrast media can be given to obtain images, thus decreasing the risk of developing contrast-induced nephropathy. Techniques developed to avoid the use of contrast media include carbon dioxide (CO2) angiography, which lowers the risk of renal toxicity, but also lowers the resolution of the image. Other complications that can result from invasive renal angiography include renal artery dissection and generation of atheroemboli.
A noninvasive method of defining the renal artery vasculature includes magnetic resonance angiography (MRA), which modifies the magnetic resonance imaging (MRI) to examine patterns of blood flow. In renal vascular disease, there is reduced blood flow distal to a stenotic lesion that causes a loss of MRA signaling. Unfortunately, loss of MRA signaling is also commonly encountered in distal vessels of normal patients thereby exaggerating the amount of narrowing found, especially in the distal half of the artery. Gadolinium MRA has been shown to significantly improve the images of the distal arteries and accessory renal arteries. Although gadolinium-enhanced MRA has previously been suggested to be an alternative non-nephrotoxic method in defining the renal artery vasculature, its use should be avoided in those with renal dysfunction due to the well-described association between gadolinium and the development of nephrogenic fibrosing dermopathy (NFD) and systemic fibrosis (NSF). Contraindications to the use of MRA include patients with claustrophobia and those with metallic implants/foreign bodies. MRA may not be as useful in detecting FMD as compared to renal angiography due to the increased spatial resolution of MRA (1 mm versus 200 νm).
High quality images, as well as three-dimensional images, of the renal arteries can be obtained using this technique. By using a continuously rotating tube and an advancing table, images can be obtained in less than 1 minute, thereby significantly reducing motion artifact. Unfortunately, as with conventional renal angiography, contrast media must be administered and therefore the concerns for nephrotoxicity exist.
Over the years, the noninvasive renal ultrasound has replaced intravenous pyelography (IVP) as the preferred method of obtaining information regarding the assessment of kidney size. Direct visualization of the renal arteries can be obtained (by B-mode imaging) and measurement of hemodynamic factors can be achieved by pulse Doppler ultrasound. Duplex ultrasound scanning is generally reported as lesions causing 0–59% stenosis and those causing 60–99% stenosis. Areas that are 0–59% stenosed are unlikely to cause lesions that are hemodynamically significant, whereas lesions that are 60–99% stenosed indicate a further need for investigation. A drawback to duplex ultrasound scanning is that the procedure is operator dependent due to its high technical demands.
Renal resistive indices are also provided by duplex ultrasound. RI approximates the amount of renal arterial impedance. In patients with RAS, an increase in renal resistive index >80% is associated with poorer postrevascularization outcome as well as an increased risk of progressive renal dysfunction.
By comparing PRA at baseline and at 1 hour after an oral dose of 25–50 mg of captopril (a short-acting ACE inhibitor), the predictive value of PRA may be increased and used to discriminate essential hypertension from RVHT. Studies have shown larger elevations in PRA and greater reductions in BP after captopril in patients with RVHT compared to those with essential hypertension (see Table 42–5). Major limitations to the widespread use of this screening method include decreased reliability of the test in patients with renal dysfunction, inability to determine unilateral versus bilateral disease, nonstandardized assay methodology of PRA between institutions, and improper patient preparation prior to testing. Ideally patients should ingest dietary salt ad libitum and hold antihypertensive medications for 2 weeks (especially ACE inhibitors, diuretics, and β-blocking agents) prior to the captopril PRA testing.
Stimulated plasma renin activity of 12 ng/mL or more |
Absolute increase in PRA of 10 ng/mL/hour or more |