Fig. 16.1
a Port placement for robotic gastrectomy. b Docked robot arms
Liver Retraction and Intraoperative Tumor Localization
Liver retraction is critical for clear visualization of the operative field. There are various liver retraction methods for upper gastrointestinal surgery: an example is the so-called liver suspension with suture-gauze technique (Fig. 16.2a) [18–20]. Regardless of the method chosen, the area around the hepatoduodenal ligament, lesser omentum and gastroesophageal junction must be exposed. In cases of distal subtotal gastrectomy , intraabdominal tumor localization is required before docking of the robotic arms [17]. To do so, metallic surgical clips are roughly placed along the greater and lesser curvatures of the stomach to demarcate the location of gastric transection. The ultimate resection line should be determined after comparing the locations between the surgical clips and intragastric hemoclips placed around the lesion during preoperative endoscopy (Fig. 16.2b).
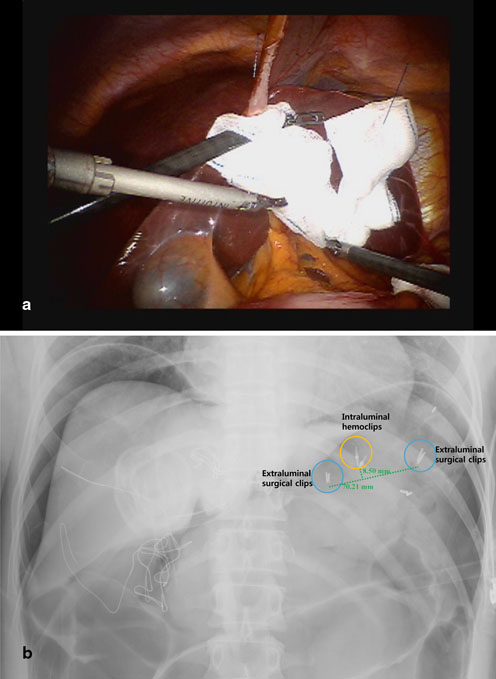

Fig. 16.2
a Liver retraction with a suture-gauze technique b Intraoperative X-ray for measuring the distance between surgical clips and intraluminal hemoclips. In this case, total gastrectomy was required
D2 Lymphadenectomy During Distal Subtotal Gastrectomy
Mobilization of the Greater Omentum
To divide the gastrocolic ligament and retrieve LN stations No. 4sb and 4d, surgeons should first retract the stomach by pulling the omentum in the direction of the patient’s head via Cadiere forceps placed in the No. 3 arm. With the use of the energy devices (ultrasonic shears or monopolar scissors) in the No. 2 arm, the lesser sac can be opened by dividing the gastrocolic ligament along the mid-transverse colon (Fig. 16.3a). The greater omentum can then be further divided toward the lower pole of the spleen. To facilitate optimal exposure of the lesser sac, proper repositioning of the omentum via the Cadiere forceps and counter traction of the transverse colon via the Maryland forceps in the No. 1 arm is essential. By this method, the left gastroepiploic vessels can be easily identified and ligated at the root using clips (Fig. 16.3b). D2 LN dissection usually comprises total omentectomy; however, partial omentectomy is an option in cases of cT1 or cT2 tumors. After clearing LN station No. 4sb, soft tissue along the greater curvature of the stomach from the imaginary proximal resection line is to be cleared to the point just distal to the short gastric vessel to complete left side LN dissection.
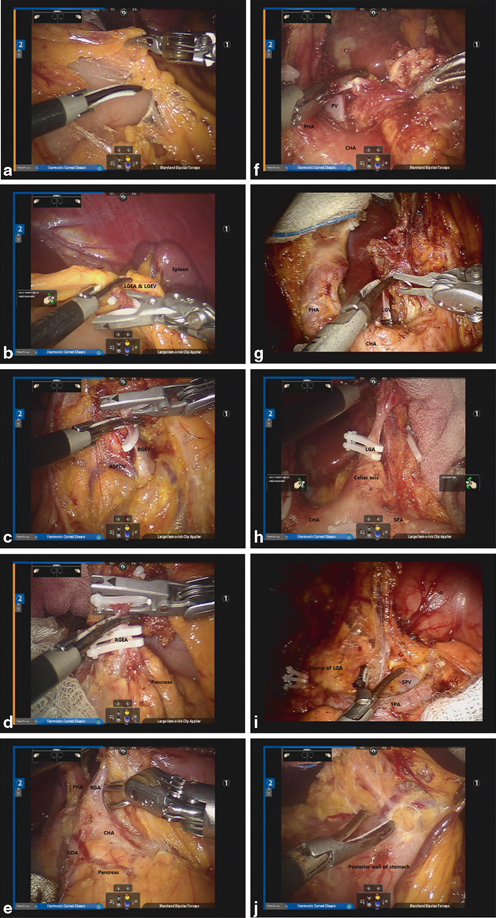

Fig. 16.3
a Division of the gastrocolic ligament. b Ligation of the Left gastroepiploic artery (LGEA) and Left gastroepiploic vein (LGEV). c Exposure of the Right gastroepiploic vein (RGEV) and ASPDV and ligation of the RGEV. d Isolation and ligation of the RGEA. e Exposure and ligation of the RGA. f Dissection of the hepatoduodenal ligament. g Isolation and ligation of the Left gastric vein (LGV). h Isolation and ligation of the LGA. i Dissection along the SPA. j Dissection of the lesser omentum along the lesser curvature
Infrapyloric Dissection
To complete infrapyloric dissection, relevant vascular anatomy around the head of the pancreas and duodenum should be well understood. Facilitated by the No. 3 arm, the distal stomach can be mobilized anteriorly from the head of the pancreas. LN bearing soft tissues, comprising station No. 6, which is bordered by the right gastroepiploic vein, the anterior superior pancreaticoduodenal vein (ASPDV), and the middle colic vein, should be accurately identified and dissected. Ligation of the right gastroepiploic vein is to be completed at the point where it joins the ASPDV (Fig. 16.3c). Subsequently, the right gastroepiploic artery (RGEA) can be identified at the end of the gastroduodenal artery (GDA) and ligated at the root thereof (Fig. 16.3d). Continued dissection will allow for identification of the infrapyloric artery in most cases, which should be isolated and ligated. Thereafter, the attachment between the posterior wall of the duodenum and the pancreas can be cleared up to the root of the GDA.
Suprapyloric Dissection and Duodenal Transection
To prevent undesired injury to the common hepatic artery (CHA) or pancreatic parenchyma during suprapyloric dissection, 4 × 4 gauze should be inserted in the space between the posterior duodenum and the head of the pancreas. Soft tissues can then be cleared just above the pylorus to a distal point about 2 cm thereto to make a path for a linear stapler. Then, the duodenum can be transected by the assistant surgeon using the linear stapler.
Suprapancreatic Dissection
After dividing the duodenum, the right gastric vessels are to be retracted to the patient’s left to generate proper tension on the vessels. Dissection is then started from the right lateral surface of the proper hepatic artery (PHA), possibly led by the GDA, which was previously exposed. The right gastric artery can then be identified and ligated at the origin (Fig. 16.3e). This completes the retrieval of LN station No. 5. By dissecting anteriorly and medially to the PHA, LN station No. 12a can be cleared. Complete dissection of LN station No. 12a can be ensured by exposing the portal vein (PV) (Fig. 16.3f). At this time, the assistant surgeon could retract the CHA inferiorly or the PHA toward the patient’s right to facilitate better exposure. Thereafter, the dissection is to continue around the CHA for the retrieval of LN station No. 8a. The left gastric artery (LGA) can then be gently retracted to the anterior abdominal wall to expose the lesser curvature. Next, the left gastric vein should be identified and securely ligated at the point where it drains into the PV or splenic vein (SPV) (Fig. 16.3g). Continuing the retroperitoneal dissection, remove the soft tissues along the LGA, celiac axis, and splenic vessels, which are designated as LN station Nos. 7, 9, and 11p, respectively. Once exposed, the root of the LGA can be ligated (Fig. 16.3h). Continued dissection of soft tissues toward the celiac trunk will allow for retrieval of LN station No. 9. Next, LN station No. 11p bearing soft tissues can be removed from the superior border of the pancreas and splenic artery (SPA) toward the middle of the SPA. Typically, a posterior gastric artery is present and can act as a landmark of the dissection border. To complete dissection of LN station No. 11p, thorough exposure of the anterior and superior borders of the SPA and SPV is generally suggested (Fig. 16.3i).
Lesser Omentum Dissection and Gastric Transection
Next, the lesser omentum along the lesser curvature from the esophageal crus down to the gastric resection line can be dissected and cleared (Fig. 16.3j). Truncal vagotomy is performed at this time by dividing the vagus nerves, which runs anteriorly and posteriorly around the esophagus. This completes dissection of LN station No. 1. After the stomach is fully mobilized, by clearing the area around LN station No. 3, it can be transected by the assistant surgeon using two or three linear staplers, completing the D2 lymphadenectomy for distal subtotal gastrectomy .
D2 Lymphadenectomy During Total Gastrectomy
Total gastrectomy with D2 LN dissection involves LN removal around distal splenic vessels (LN station No. 11d) and the splenic hilum (LN station No. 10) with or without splenectomy. To prevent increased morbidity related to the splenectomy, spleen-preserving total gastrectomy with D2 LN dissection is possible. However, splenic hilar dissection for the purpose of spleen-preservation is a highly-demanding procedure, although experienced surgeons can perform it successfully. Notwithstanding, use of magnified 3D views from the robotic system and the articulated movements of the robotic arms allows for this complex and difficult procedure to be more readily performed. The detailed procedures thereof include dissection of LNs around the distal splenic vessels and the splenic hilum. To begin, the short gastric vessels are divided after ligating the left gastroepiploic vessels. After division of the short gastric vessels at their roots, the esophagophrenic ligament is divided for mobilization of the esophagus. Subsequently, branches of the splenic vessels can be identified and ligated from the lower pole toward the upper pole of the spleen and from the splenic hilum toward proximal part of the splenic vessels (Fig. 16.4a and b).
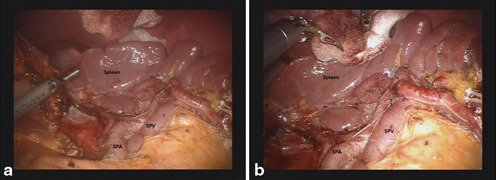

Fig. 16.4
a Complete dissection along the splenic artery and hilum during spleen-preserving total gastrectomy. b Completion of splenic hilar dissection
Reconstructions
Depending on the location of tumors or the size of remnant stomach after distal subtotal gastrectomy, bowel continuity can be restored by gastroduodenostomy (Billroth I) and gastrojejunostomy (Billroth II). Roux-en-Y gastrojejunostomy is also worth considering. In instances of total gastrectomy, Roux-en-Y esophagojejunostomy is routinely performed. Intracorporeal stapled anastomosis can be used for totally robotic gastrectomy. Robotic sutured anastomosis is also feasible [21]. Up to now, however, stapling has been carried out by the assistant surgeon during robotic surgery. There are several reconstruction methods used during laparoscopic distal subtotal or total gastrectomy that can also be applied for robotic surgery [22–26]. Herein, a few of these intracorporeal anastomosis techniques after robotic distal subtotal and total gastrectomy are described.
Intracorporeal Gastroduodenostomy (Billroth I)
Intracorporeal gastroduodenostomy is the first option for restoration of intestinal continuity after distal subtotal gastrectomy , whenever indicated [27]. Using linear staplers, surgeon can perform gastroduodenostomy also known as delta-shaped anastomosis [25, 27]. To do so, the duodenum should be transected from the posterior to anterior wall using a 45-mm linear stapler. After gastric transection, a small entry hole in the remnant stomach is created along the resection line of the greater curvature. The medial edge of the duodenal stump can also be opened to create an entry hole. Using a 45-mm linear stapler, a common channel is formed between the posterior wall of the stomach and the posterior wall of the duodenum after which the entry hole can be closed by another two 45-mm linear staplers. Unlike conventional delta-shaped anastomosis, there are some modifications. Some surgeons prefer to remove the previous stapling line of the duodenum, when closing the entry hole. During the procedure, linear staplers can be inserted and used via the assistant port.
Intracorporeal Gastrojejunostomy (Billroth II)
When gastroduodenostomy is not indicated after distal subtotal gastrectomy, intracorporeal gastrojejunostomy can be performed by antecolic, isoperistaltic loop gastrojejunostomy [22, 27]. After complete resection, the remnant stomach is measured and the jejunal loop is mobilized in order to make entry holes for a linear stapler. Thereafter, an entry hole in the stomach is created at the greater curvature about 6 cm apart from the previous transection line. Sometimes a short gastric artery may need to be sacrificed. Then, an entry hole in the loop of the jejunum 15–20 cm away from the ligament of Treitz is created at the antimesenteric border. A 60-mm-sized linear stapler can then be introduced from an assistant port on the left side and accommodated in the stomach and the jejunum one by one. The jejunum should be gently brought up around the hiatus and then, inserted gently using the robotic arms. After side-to-side anastomosis is performed, the No. 2 arm of the robot needs to be undocked and an 8-mm port should be replaced with a 12-mm trocar to facilitate use of a linear stapler for closure of the entry hole. Using a 60-mm linear stapler on the right side, the common enterotomy can be closed. The entry hole may also be closed by a robotic hand-sewn technique.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







