Fig. 4.1
H. pylori and Epstein–Barr virus infection associated gastric adenocarcinoma. a An adenocarcinoma arises in association with active chronic gastritis with H. pylori organisms identified on immunohistochemical stain ( insert). b A poorly differentiated carcinoma with intense intraepithelial and stromal lymphocytic infiltration ( arrow) and EBV genome is identified by in situ hybridization ( insert)
Epstein–Barr virus (EBV) has long been recognized as a distinct pathogenic cause of gastric carcinoma [25, 26]. EBV is detected in about 10 % of the gastric carcinoma cases (Fig. 4.1b). All tumor cells in EBV-associated gastric carcinoma harbor the clonal EBV genome. Gastric carcinoma associated with EBV occurs predominately in men and in younger-aged individuals. These carcinomas exhibit a unique histologic phenotype, genetic/epigenetic genotype, and distinct clinicopathological features [25, 27–29].
Autoimmune Gastritis
Autoimmune gastritis arises secondary to an immune-mediated destruction of parietal cells (pernicious anemia), is confined to the body and fundus of the stomach, and is characteristically associated with neuroendocrine cell (enterochromaffin-like cell) hyperplasia and neoplasia (Fig. 4.2). In patients with autoimmune associated atrophic gastritis, most adenocarcinomas are of the intestinal type and the risk of gastric cancer increases at least three fold [30]. In contrast, gastric type-1 neuroendocrine (carcinoid) tumors arising in autoimmune atrophic gastritis are relatively indolent in their behavior [31].
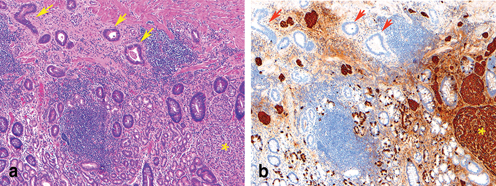
Fig. 4.2
Type-I gastric neuroendocrine tumor and the coexisting adenocarcinoma. Histopathology of a neuroendocrine tumor ( star) exhibits a nested pattern (a) and the immunoreactivity for chromogranin (b) is present in the background of hyperplastic neuroendocrine cells and neuroendocrine tumor. A well differentiated and gland-forming adenocarcinoma ( arrow) invades the muscularis mucosa and infiltrates the submucosa
Gene-Dietary Interaction
Environmental factors in addition to H. pylori infection, including cigarette smoking and diet, play an important role in gastric carcinogenesis [32]. Foods that are salted, smoked, pickled, and preserved foods rich in salt, nitrites, or preformed N-nitroso compounds are associated with an increased risk of gastric cancer [33].
Genetic polymorphisms may also contribute to the etiology of gastric cancer by altering the activity of enzymes that are involved in multiple molecular processes, such as DNA synthesis and repair, carcinogen metabolism, the inflammatory response, and tumor suppression [34]. Individuals who carry high-risk genetic variants and high-risk diets have an increased risk of gastric cancer compared with those who do not carry high-risk genetic variants or those with high-risk genetic variants but low-risk diets. Distinctive dietary patterns and regional variations in genetic polymorphisms may explain regional variations in gastric cancer incidence [35–37].
Hereditary
Approximately 10 % of all gastric cancers are familial. Germline mutations in the E-cadherin CDH1 gene account for 30–40 % of the rare syndrome known as hereditary diffuse gastric cancer, and gastric cancers also occur less frequently as a component of other hereditary cancer syndromes [38].
Familial Diffuse Gastric Carcinoma
Germline mutations in CDH1 are the molecular basis for familial gastric cancer syndrome [39–42] (Fig. 4.3a). Initially identified in three Maori families in New Zealand, at least 100 families have been reported to carry the CDH1 germline mutation [43]. Given the relatively high penetrance disease (70–80 %) [44], a lifetime risk of developing gastric cancer of approximately 67 % in men and 83 % in women [45], prophylactic total gastrectomy is often considered after a familial diagnosis of a CDH1 mutation [46]
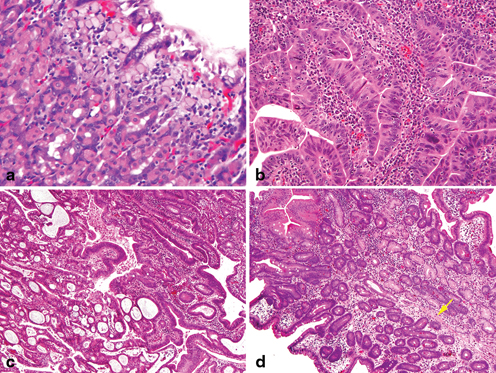
Fig. 4.3
Hereditary condition associated gastric neoplasms. a Early hereditary diffuse gastric carcinoma with signet ring cell morphology is present in the superficial lamina propria. b HNPCC (Lynch syndrome) associated intestinal type gastric adenocarcinoma exhibits increased intraepithelial and stromal lymphocytes. c An FAP associated adenocarcinoma ( left) arises in a fundic gland polyp with dysplasia ( upper right). d Gastric Peutz–Jeghers polyp is composed of irregular and architecturally distorted proliferation of foveolar glands with increased inflammation in the lamina propria and smooth muscle proliferation ( arrow)
Hereditary Nonpolyposis Colorectal Cancer (HNPCC) Syndrome
After endometrial carcinoma, gastric carcinoma is the second most common extra-colonic cancer in patients with hereditary nonpolyposis colorectal cancer (HNPCC) (Fig. 4.3b). There is a four-fold relative risk of developing gastric cancer in HNPCC patients, with the risk predominantly in younger patients (11.3-fold in the 30s and 5.5-fold in the 40s). Additionally, the relative risk is greater in mutation carrier families than noncarrier families (3.2-fold versus 1.6-fold). The overall lifetime risk of developing gastric cancer is 10 % for patients of Western ancestry and 30 % for patients of Asian ancestry [54–57], and microsatellite instability (MSI) phenotype is noted in 65 % of these cases.
Familial Adenomatous Polyposis Coli (FAP)
Patients with familial adenomatous polyposis coli (FAP) also develop multiple gastric fundic gland polyps, which can undergo neoplastic transformation as a result of somatic mutations of the adenomatous polyposis coli (APC) gene [47] (Fig. 4.3c). However, in contrast to the development colon adenocarcinoma from adenomatous polyps in FAP patients, the development of gastric carcinoma in fundic gland polyps is rare [48–51]. Interestingly, there is a higher risk of neoplastic transformation in the stomach of Asian FAP patients as compared to Western FAP patients [52, 53] .
Li–Fraumeni Syndrome
Germline mutations of the TP53 gene are present in 50–70 % of the patients with Li–Fraumeni syndrome. The most common neoplasms in patients with Li–Fraumeni syndrome are soft tissue sarcoma, breast cancer, and brain tumors. While gastrointestinal tract tumors account for less than 10 % of all Li–Fraumeni syndrome associated neoplasms, gastric carcinomas (which may be multiple) represent more than 50 % of the gastrointestinal tumors in patients with Li–Fraumeni [58, 59].
Peutz–Jeghers Syndrome
Mutation of the serine/threonine–protein kinase 11 (STK11) gene , located on chromosome 19p13.3, is responsible for Peutz–Jeghers syndrome [60]. Characteristic gastrointestinal hamartomatous polyps develop (Fig. 4.3d), and these patients have an increased risk of gastric cancer, although the exact degree of risk is a subject of debate [61, 62].
Gastric Hyperplastic Polyposis
Precursors of Gastric Carcinoma
The well-defined chronic inflammation-intestinal metaplasia-glandular dysplasia—cancer sequence typically precedes the development of most intestinal type gastric adenocarcinomas [65]. While intestinal metaplasia proceeded by epithelial dysplasia (type I) may be present as a polypoid lesion and resemble a colonic adenoma, it is genetically distinct from the typical tubular adenoma in the colon. In contrast to adenoma-carcinoma sequence in colonic adenocarcinoma (which is usually associated with an intrinsic genetic abnormality in the APC molecular pathway) the progression of intestinal dysplasia to gastric adenocarcinoma occurs with a stepwise accumulation of multiple genetic abnormalities. True de novo gastric adenomas are rare outside the setting of FAP, in which gastric fundic gland polyps progress to epithelial dysplasia secondary to inherent APC gene abnormality. A less common histologic variant of dysplasia is gastric foveolar (type II) dysplasia with a gastric mucin phenotype [66]. The significance of these subtypes remains controversial and phenotyping of gastric dysplasia is not recommended at this time.
The natural history of gastric dysplasia depends on its grade, extent of dysplasia, and surface appearance (polypoid versus flat or depressed). Dysplasia is graded based on cytologic and architectural features as either low grade (LGD) or high grade (HGD) (Fig. 4.4a, b). Low-grade dysplasia diagnosed on endoscopic biopsies has been shown to regress in 38–75 % of the cases, to persist in 19–50 %, and to progress to HGD in 0–9 % of the cases [67]. The best independent predictors of progression to adenocarcinoma are lesions greater than 2 cm and a depressed configuration on endoscopic examination [68].
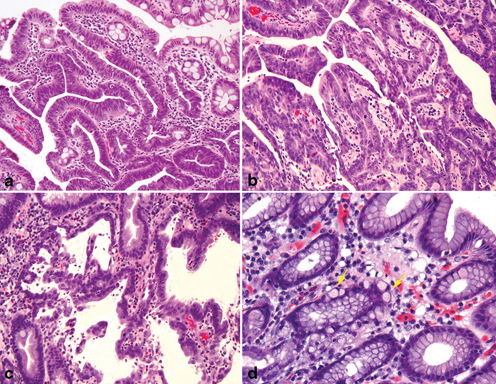
Fig. 4.4
Precursors of gastric adenocarcinoma. a Long standing chronic gastritis is followed by intestinal metaplasia ( upper right) and low-grade glandular dysplasia which is demonstrated by nuclear elongation and pseudostratification. b High-grade dysplasia exhibits loss of cellular polarity of the epithelium with glandular crowding and architectural alteration which approaches the criteria of early carcinoma. c Even in the absence of invasion into the stroma, early adenocarcinoma proceeded from high-grade dysplasia is demonstrated by expansile crypt growth with cribriform complexity. d In situ signet ring cell carcinoma is present within the basal membrane with hyperchromatic and depolarized nuclei and pagetoid spread of signet ring cells ( arrow)
High-grade dysplasia regresses in only 0–16 % of the cases, persists in 14–58 %, and progresses in 10–100 % to adenocarcinoma (Fig. 4.4c) [67]. Given the high probability of progression to adenocarcinoma, a lesion diagnosed as HGD on endoscopic biopsy should be considered for endoscopic mucosal resection if feasible or surgical resection if HGD is present as multifocal lesions or if endoscopic mucosal resection is not technically feasible.
The precursor of diffuse gastric carcinoma is thought to originate from oxyntic gland tubule neck (or globoid) dysplasia [69] in situ signet ring cell carcinoma. This corresponds to the presence of signet ring cells within the basal membrane, generally with hyperchromatic and depolarized nuclei and pagetoid spread of signet ring cells below the preserved epithelium of glands/foveolae (Fig. 4.4d) [70] .
Pathologic Classification
Tumor Location
The location of gastric adenocarcinoma may, to some extent, reflect the pathogenesis of the disease. For example, intestinal type adenocarcinoma in the proximal stomach may be associated with a reflux etiology (Fig. 4.5a), while intestinal type adenocarcinoma in the distal stomach is more likely related with H. Pylori infection associated pathogenesis (Fig. 4.5b). Diffuse type gastric cancer is more commonly located in the middle third and body of the stomach (Fig. 4.5c), while remnant cancer is invariably located in the gastric mucosa at duodenogastric anastomosis (Fig. 4.5d). Determination of a precise tumor location can be challenging and even subjective, especially when the lesion is large and straddles multiple anatomical sites within the stomach. Nevertheless, documentation of the relative location of the tumor is important for the elucidation of potential pathogenesis and classification of the disease, as well as for the evaluation of the extent of the disease and the resection margin status .
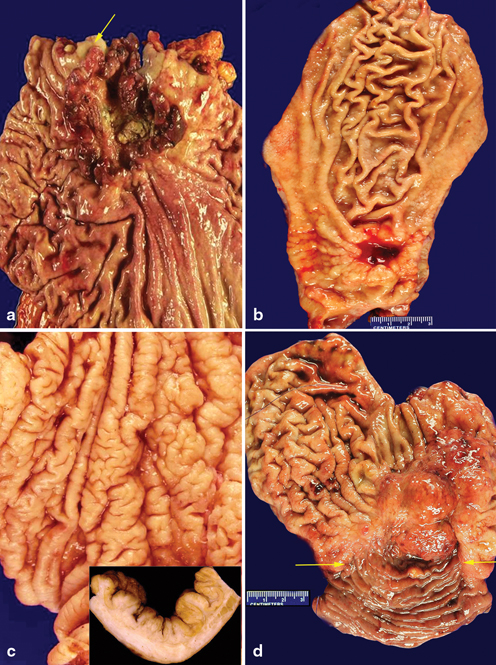
Fig. 4.5
Gross pathology of gastric adenocarcinoma. a A proximally located gastric adenocarcinoma with minimal extension into the squamous mucosa ( arrows) of the esophagus. b An ulcerated intestinal carcinoma is located in the distal stomach. c A diffuse type adenocarcinoma is located in the body of the stomach with intact mucosa but rigid mucosal fold. A cross section of the mucosa reveals thickened gastric wall secondary to diffuse infiltration by tumor cells. d A remnant gastric cacumina is located in the gastric mucosa near the anastomotic line ( arrows)
Gross Pattern
The gross configuration of advanced gastric cancer can be classified using Borrmann classification, which designates gastric carcinomas into four distinct types[71]: polypoid (type I), fungating (type II), ulcerating (type III), and diffusely infiltrating (type IV). Diffusely infiltrating is also referred to as linitis plastica when it involves nearly the entire stomach and it is consistently associated with the diffuse histologic subtype. In contrast, types I, II, and III are associated with other histologic subtypes. Type II, the most common subtype, represents 36 % of all gastric carcinomas and is frequently detected on the lesser curvature of the antrum. Types I and III each represent 25 % of all advanced gastric carcinomas, and they are more common in the corpus, usually on the greater curvature.
Histologic Classification
Gastric cancer represents a heterogeneous group of tumors with diverse pathogenesis, morphologic features, and molecular backgrounds. While recent genomic analysis has identified several subtypes of gastric adenocarcinoma by their generic signatures [29], histopathologic classification remains critical for a number of clinical assessments of the disease and serves as the basis for the molecular classification of the disease [72, 73]. Several systems have been proposed to aid in the classification of gastric adenocarcinoma based on the microscopic futures of the tumor [74–76]. The two most commonly used histologic classifications are the Laurén classification and the World Health Organization (WHO) systems [77, 78]; significant correlation is seen between these two schemes [79].
The Laurén classification separates gastric adenocarcinomas into two primary subtypes: intestinal and diffuse, and tumors exhibiting features of both the intestinal and diffuse types are designated as mixed-type adenocarcinoma (Fig. 4.6a, b, c, d). The intestinal type is characterized by the formation of glands exhibiting various degrees of differentiation either with or without extracellular mucin production (Fig. 4.6a). The diffuse type of gastric adenocarcinoma is composed of poorly cohesive cells without gland formation (Fig. 4.6b, c). This type of tumor often contains cells with intracytoplasmic mucin, known as “signet ring cells ” (Fig. 4.6c), although this term has been synonymously used for diffuse cancer even in the absence of intracytoplasmic mucin (Fig. 4.6c). In addition to their distinct morphologic characteristic, the intestinal and the diffuse subtypes of gastric adenocarcinoma also have different clinicopathologic features (Table 4.1) .
Table 4.1
Clinical and pathologic features of Laurén subtype gastric adenocarcinoma
Intestinal type | Diffuse type | |
|---|---|---|
Onset age | Older than 50 year | Younger than 50 years |
Gender | Male > Female | Male = Female |
Geographic distribution | Asia (China Japan, Korea) | Anywhere |
Precursor lesion | Intestinal metaplasia/dysplasia | Signet ring cell carcinoma in situ |
Common location | Antrum or cardia | Body |
Borrmann classification | Type I, II, III | Type IV |
Genetic association | HNPCC, AFP | Hereditary diffuse gastric cancer, hyperplastic polyposis |
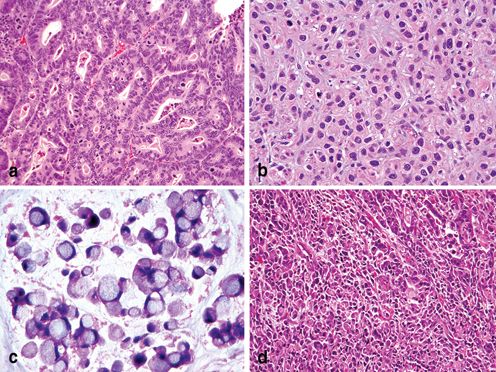
Fig. 4.6
Lauren’s histopathology classification of Gastric Carcinoma. a Intestinal type adenocarcinoma with wellformed glandular and tubular architecture. b Poorly differentiated diffuse type adenocarcinoma. c Diffuse type adenocarcinoma with intracellular mucin and signet ring cell features. d Lauren’s mixed type adenocarcinoma with a small component of poorly differentiated intestinal phenotype ( upper right) and a poorly differentiated diffuse/poorly cohesive carcinoma with focal signet ring cell features ( left)
While the basis for the initial Laurén classification was exclusively morphologic characteristics, accumulative knowledge in the epidemiology and pathogenesis of gastric carcinoma has indicated that this classification system is also valuable in defining molecular subtypes of gastric cancer [72, 73]. In the absence of significant chronic gastritis, intestinal metaplasia, or dysplasia, pure diffuse type of gastric cancer probably represents either a hereditary or sporadic ideology. However, significant components of diffuse or poorly cohesive carcinoma can be seen in mixed adenocarcinoma with inflammation-metaplasia-dysplasia-carcinoma precursors, often complicating molecular analysis of the tumor.
In 2010 the WHO revised its morphologic classification to reflect the patterns exhibited throughout the gastrointestinal (GI) tract [78]. This classification recognizes five major types of gastric adenocarcinoma based on the predominant histologic growth pattern: (1) papillary, (2) tubular, (3) mucinous (tumors with mucinous pools exceeding 50 % of the tumor), (4) poorly cohesive (including signet ring cell carcinoma and other variants), and (5) mixed adenocarcinomas (Table 4.2). Uncommon variants of gastric carcinomas include the squamous cell, adenosquamous, hepatoid (Fig. 4.7a), micropapillary, carcinoma with lymphoid stroma (medullary carcinoma) (Fig. 4.7b), carcinoma with pancreatic acinar differentiation (Fig. 4.7c), choriocarcinoma [80, 81], undifferentiated subtypes (Fig. 4.7d), carcinoma with sarcomatous differentiation (Fig. 4.7e), high grade neuroendocrine carcinoma of small cell or large cell subtype (Fig. 4.7f), and carcinoma arising in gastric heterotopia in the esophagus (gastric inlet) or pancreatic heterotopia. The so called medullary carcinoma usually has an expansile growth pattern with intratumoral and peritumoral lymphocytic infiltration; this tumor phenotype is commonly associated with either EBV or microsatellite instability associated gastric carcinoma. The relevant clinical implication when encountering these rare subtypes of gastric carcinoma is that a metastasis should be excluded before entertaining a diagnosis of primary gastric carcinoma. In addition, any histologic subtype of gastric carcinoma, when poorly differentiated, can present with either partial or entirely sarcomatous features (sarcomatoid carcinoma) (Fig. 4.7e), which is not uncommon in the upper gastrointestinal tract or the pancreaticobiliary carcinoma.
Tumor type | Histologic features |
|---|---|
Adenocarcinoma | |
Papillary adenocarcinoma | Exophytic with elongated frond-like tumor extensions with fibrovascular cores; usually better differentiated and low grade |
Tubular adenocarcinoma | Dilated or slit-like branching tubules; usually low, although poorly differentiated variants are not uncommon |
Mucinous adenocarcinoma | Contains more than 50 % extracellular mucin pools. May contain scattered signet-ring cells more commonly seen in proximal/cardia location |
Poorly cohesive carcinomas, including diffuse and signet-ring cell carcinoma and other variants | Tumor cells infiltrate as isolated single cells or small aggregates. The carcinoma is predominantly composed of signet-ring cells containing a clear droplet of cytoplasmic mucin displacing the nucleus. Other variants of poorly cohesive carcinoma may resemble mononuclear inflammatory cells |
Mixed carcinoma | Mixture of morphologically identifiable components such as tubular, papillary, and poorly cohesive patterns |
Adenosquamous carcinoma | Mixture of glandular and squamous neoplastic components; the squamous component should comprise at least 25 % of the tumor volume |
Carcinoma with lymphoid stroma (medullary carcinoma) | Poorly developed glandular structures associated with a prominent lymphoid infiltrate in the stroma. Associated with EBV infection or HNPCC-associated carcinoma and may have a favorable prognosis |
Hepatoid adenocarcinoma | Large polygonal eosinophilic tumor cells resembling hepatocytes; may express alpha-fetoprotein |
Squamous cell carcinoma | Both Keratinizing and nonkeratinizing forms are encountered |
Undifferentiated carcinoma | High-grade carcinoma that cannot be further classified as adenocarcinoma, squamous cell carcinoma, or other recognized variants |
Neuroendocrine carcinoma | Poorly differentiated high-grade carcinoma with diffuse or focal synaptophysin chromogranin-A expression. These tumors exhibit a high mitotic rate (> 20 per 10 high power field, and Ki67 is usually > 50 %) marked nuclear atypia, and may have focal necrosis |
Large cell neuroendocrine carcinoma | Tumor cells are large, with moderate amount of cytoplasm, and may contain prominent nucleoli |
Small cell neuroendocrine carcinoma | Tumor cells are small, with finely granular chromatin and indistinct nucleoli |
Mixed adenoneuroendocrine carcinoma | Composed of both gland-forming and neuroendocrine malignant elements, with at least 30 % of each component. Identification of scattered neuroendocrine cells in adenocarcinomas by immunohistochemistry does not qualify as mixed carcinoma |
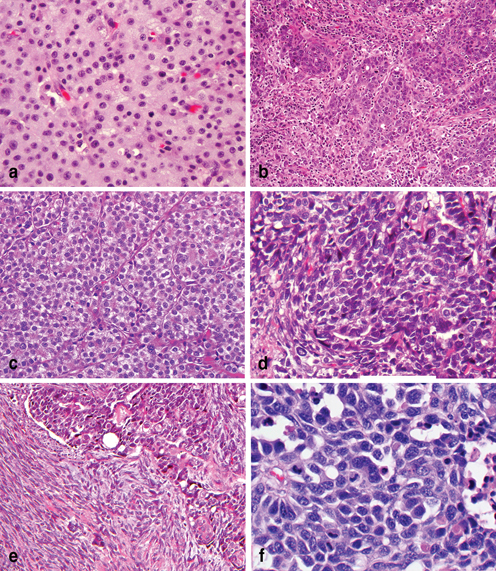
Fig. 4.7
Uncommon histopathologic variants of gastric adenocarcinoma. a Adenocarcinoma with hepatoid features. b Medullary adenocarcinoma with markedly increased intraepithelial and stroma lymphocytes ( small blue cells). c Adenocarcinoma with prominent pancreatic acinar differentiation. d Undifferentiated carcinoma. e Undifferentiated carcinoma ( upper right) with sarcomatous differentiated ( low left). f High grade neuroendocrine carcinoma, small cell type
Diagnostic Issues
Primary Versus Metastasis
The pathologic diagnosis of gastric adenocarcinoma, particularly a poorly differentiated and nonintestinal subtype, can be challenging with a biopsy specimen. While stomach is not a common site for metastasis, a number of epithelioid neoplasms can metastasize to the gastric mucosa and the differential diagnosis between a primary gastric carcinoma and a metastasis may be difficult in small biopsies [82, 83]. Patients may be asymptomatic, present with a bleeding ulcer mimicking a primary gastric carcinoma (39 % of the cases), or with a submucosal tumor (51 % of the cases).
The most commonly observed error in the diagnosis of diffuse signet ring cell carcinoma occurs with metastatic lobular breast carcinoma, which has a propensity to metastasize and colonize the gastrointestinal tract as well as other hollow organs such as the uterus and the urinary bladder. Primary gastric diffuse signet ring cell carcinoma and lobular breast carcinoma share similar morphologic features and sometimes, the two neoplasms can be indistinguishable on the morphologic basis alone (Fig. 4.8a, b). Immunohistochemical studies can be helpful, since classic lobular breast carcinoma is usually immunoreactive to estrogen receptor (ER) (Fig. 4.8c), cytokeratin-7 (CK7), and mammaglobin; and a gastric primary carcinoma is immunoreactive for both CK7 and CK20, and should be negative for ER and mammaglobin .
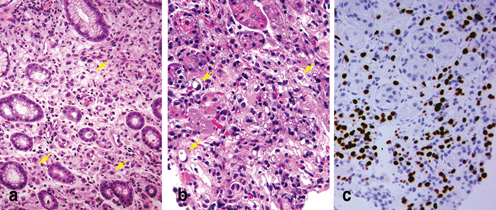
Fig. 4.8
Differential diagnosis of diffuse carcinoma in gastric biopsies. Primary diffuse gastric carcinoma (a) and metastatic breast lobular carcinoma to the stomach (b) share morphologic features ( Arrow) and the distinction between them may sometimes be impossible. An immunostain for estrogen receptor is usually positive in classic lobular carcinoma (c)
Most importantly, a clinical history, even in the remote past, of breast carcinoma should prompt the appropriate work up to exclude a metastasis before the diagnosis of primary gastric diffuse signet ring cell carcinoma. Female patients with hereditary CDH1 mutation are at risk of developing both diffuse type gastric adenocarcinoma and lobular breast carcinoma, although the reported incidence of the latter is lower [45].
Gastrointestinal stromal tumor (GIST) can occur at any site of the GI tract; the stomach is one of the most common locations. When a GIST has epithelioid morphology, it can be difficult to distinguish from a poorly differentiated primary gastric carcinoma. Although subtle morphologic details may suggest the diagnosis of a GIST, such as intercellular myxoid stroma (Fig. 4.9a), a lack of cytokeratins immunoreactivity and positive reactivity to c-kit (CD117) confirms a diagnosis of GIST (Fig. 4.9b).
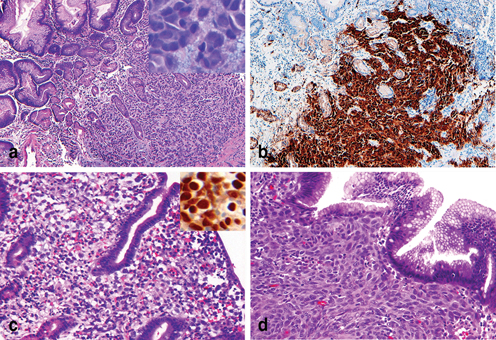
Fig. 4.9
Differential diagnosis of poorly differentiated epithelioid neoplasms in gastric biopsies. a Epithelioid gastrointestinal stromal tumor (GIST) involves gastric mucosa; the tumor cells exhibit intercellular myxoid stromal ( insert) which is a subtle feature of GIST. b An immunostain of c-KIT (CD117) can confirm the diagnosis of GIST. c Metastatic seminoma involving gastric mucosa and an immunostain of octamer-binding transcription factor 4 (OCT4) ( insert) is usually positive in tumor cells. d Metastatic melanoma involving gastric mucosa
Other poorly differentiated malignant epithelial or epithelioid tumors, including seminoma (Fig. 4.9c), melanoma (Fig. 4.9d), and renal cell carcinoma, can metastasize to the stomach. Therefore, a poorly differentiated neoplasm in a gastric biopsy requires a thorough clinical and pathologic evacuation to exclude the possibility of a metastasis before the establishment of a primary gastric cancer. Among metastatic glandular/tubular carcinomas, pulmonary and pancreatic origins are more common than other primaries .
Biopsy Diagnosis of Early Gastric Cancer
Adenocarcinoma confined to the gastric mucosa (pathologic stage pT1a) or submucosa (pT1b) is defined as early gastric cancer (EGC) [7], and represents an early stage in tumor development. In Western series, EGC represents 15–20 % of the newly diagnosed gastric cancers, whereas in Japan it accounts for more than 50 % of the cases [2–5]. A higher prevalence of gastric cancer, more liberal use of upper endoscopy and chromoendoscopy, and differences in diagnostic criteria may explain the differences between Western and Japanese studies.
Most EGCs are typically located on the lesser curvature, around the angularis, and majority of them are well differentiated tubular or papillary variants [7]. These features create a challenging differential diagnosis between high-grade glandular dysplasia/carcinoma in situ (pTis) (Fig. 4.10a), and minimally invasive carcinoma (pT1a). The latter may present as either (1) individual cribriform glands with an associated expansile growth pattern (Fig. 4.10b) or (2) with nominal tumor invasion in the lamina propria (Fig. 4.10c); in both histologic prototypes, the tumor has progressed beyond the level of glandular dysplasia and met the diagnostic criteria of superficial gastric adenocarcinoma. When carcinoma invades through the muscularis mucosa, the tumor is staged as pT1b (Fig. 4.10d). Diffuse-type EGCs tend to exhibit greater width and depths of invasion and thus are less challenging to diagnose.
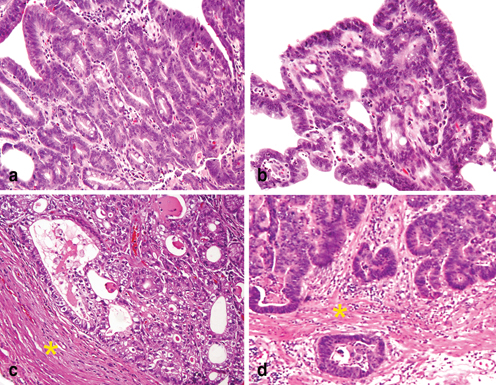
Fig. 4.10
Biopsy diagnosis of early gastric cancer . a High-grade glandular dysplasia with crowded glands in the superficial lamina propria is staged as in situ carcinoma (pTis). b An example of early gastric adenocarcinoma which exhibits expansile and complex glandular architecture, thus the lesion has progressed beyond high-grade dysplasia. Although stromal invasion cannot be assessed in this superficial biopsy, the tumor should be staged as pT1a. c Adenocarcinoma with extensive lamina propria invasion, but the tumor is confined to the mucosa without muscularis mucosae (marked by *) invasion and is staged as pT1a. d Adenocarcinoma has invaded thought the muscularis mucosae (marked by *) and into the superficial submucosa, and the tumor is staged as pT1b
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







