At-risk conditions
Endoscopy surveillance recommendations
Pernicious anemia
At time of diagnosis, UGI WLE for increased risk of gastric cancer and type I carcinoids. Subsequent surveillance interval unclear
Partial gastrectomy
At 15–20 years after surgery, UGI WLE with biopsies of the gastric remnant and anastomosis. Subsequent surveillance interval unclear
Hereditary diffuse gastric cancer
Beginning at 10 years earlier than the youngest affected family member or by 25 years of age, UGI WLE, every 6 months to 1 year, with six random biopsies each at the fundus, cardia, body, body-antral transition, and antrum, and targeted biopsies of any endoscopically visible lesions
Peutz-Jeghers syndrome
Starting at 8 years of age using UGI WLE. If significant polyps are found, repeat surveillance endoscopy every 3 years. If no polyps are detected, next surveillance endoscopy can be delayed to 18 years of age unless symptoms arise
Juvenile polyposis syndrome
Beginning at 15 years of age with UGI WLE. Surveillance every 1–3 years unless there are interval symptoms
Familial adenomatous polyposis
Starting at 25–30 years of age for increased risk of fundic gland polyps, gastric adenomas, gastric cancer, duodenal, and periampullary adenomas and malignancies
Hereditary nonpolyposis colorectal cancer
Commencing at 30–35 years of age with UGI WLE. Subsequent surveillance at 2–3 years intervals unless symptomatic
Diagnosis
As a result of organized screening programs, up to 50 % of diagnosed gastric cancers in countries like Japan are of early stage, defined as those limited to the mucosa or submucosa regardless of lymph node involvement [40, 41]. On WLE, early gastric cancer can be subtle, and therefore gastric contents should be suctioned away, the mucosal surface thoroughly washed of bubbles or debris, and the stomach well insufflated. The endoscopist should be attentive to interruptions of the mucosal folds, differences in mucosal color, mucosal friability, spontaneous bleeding, and changes in submucosal vessel patterns [42]. When a suspected early gastric cancer is identified, its morphology, location, size, and margins should be characterized. Current indications for EMR or endoscopic submucosal dissection (ESD) include a < 2 cm nonulcerated, T1a differentiated type adenocarcinoma, with recently proposed expanded criteria [41]. Categorization of lesion morphology has been internationally standardized based on the Paris Endoscopic Classification [43]. Neoplastic lesions can be polypoid, which protrudes above the surrounding mucosa, and may have a narrow base (i.e., pedunculated) or have a base diameter similar to the top (i.e., sessile). Alternatively, nonpolypoid lesions could be slightly elevated, completely flat, or depressed compared to the surrounding mucosa [43] (Fig. 9.1). The surface morphology may also guide T staging. The findings of smooth surface protrusion or depression, slight marginal elevation, and smooth tapering of converging folds have a reported 82 % positive predictive value for T1m disease when compared to pathological staging. Conversely, an irregular surface, marked marginal elevation, and abrupt cutting/fusion of converging folds had a 72 % positive predictive value for T1sm disease. The overall accuracy of distinguishing T1m from T1sm lesion was 78 % [44]. Lesion location can be prognostic in EMR, as those at the fundus, mid/lower body or incisura, versus the antrum, were associated with higher rates of incomplete EMR in a multicenter retrospective review of over 500 EMRs performed in Korea [45]. For advanced disease amenable to gastrectomy, tumor location, particularly in relation to the esophagogastric junction and incisura, also guides the extent of surgical resection. Lesion size is likewise prognostic in EMR, with those smaller than 3 cm achieving a higher complete resection rate than larger lesions [45]. This characteristic however has become less relevant with the advent of ESD, which allows enbloc resection of large lesions, otherwise removed piecemeal by EMR. The lateral margins of relatively flat lesions can be difficult to delineate, raising the possibility of incomplete endoscopic resections. The development of chromoendoscopy and narrow band imaging (NBI), however, has enhanced margin delineation. Chromoendoscopy is a form of enhanced imaging, whereby a dye is sprayed via the working channel on both the suspected lesion and surrounding mucosa. Indigo carmine dye is not absorbed by gastric epithelium, but instead pools in crevices highlighting differences in elevation, and mucosal irregularities. Its combined use with acetic acid was superior to either alone, or WLE alone for tumor border recognition, though a more recent study showed improved visualization among well-differentiated cancers only [46, 47]. NBI is an equipment-based form of image-enhanced endoscopy, whereby an optical filter allows light of limited wavelengths, specifically blue and green light, to illuminate the mucosa, highlighting surface and vascular architecture. Use of NBI alone to survey the gastric mucosa in its entirety is impractical due to the darkness of the lumen. Its value lies in further characterizing a lesion once identified. Normal mucosa, H. pylori-associated gastric atrophy, and intestinal metaplasia have distinct microsurface and microvascular features enabling differentiation from early gastric cancers [48, 49]. For example, on magnification NBI (M-NBI), the light blue crest sign on the epithelial surface has a sensitivity and specificity of approximately 90 % for gastric intestinal metaplasia [50]. And the demarcation line which represents a transition of the microsurface and microvasculature characteristics on M-NBI is most indicative of cancer, and was concluded as useful to determine the lateral extent of early gastric cancer at an Asian-Pacific endoscopy consensus meeting [48, 49]. M-NBI may have better sensitivity and specificity than chromoendoscopy for the diagnosis of gastric cancers less than 5 mm [51]. Of note, however, is that undifferentiated early gastric cancers may spread subepithelially along the lamina propria, and have a normal overlying foveolar epithelium, thus limiting the utility of M-NBI [48]. Hayee et al. recently proposed a diagnostic algorithm for gastric epithelial lesions with WLE and M-NBI [49].
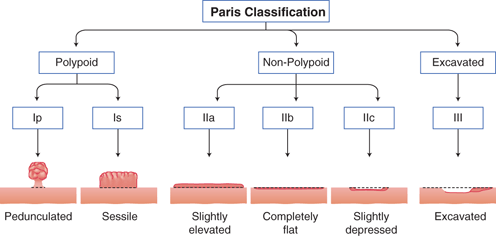

Fig. 9.1
Paris classification for superficial (type 0) neoplastic lesions of the gastrointestinal tract. Based on the endoscopic macroscopic appearance, lesions are categorized as polypoid/protruding: Ip or Is, or nonpolypoid/nonprotruding: IIa, IIb, IIc, or III
After visual characterization, 8–10 biopsies should be performed of the suspected neoplasia, particularly for ulcerated lesions, with standard size biopsy forceps [52]. Jumbo forceps may increase diagnostic yield, though a recent open-labeled study found for nonulcerated gastric epithelial lesions, four standard forceps (opening diameter 6.8 mm) biopsies and four jumbo forceps (opening diameter 8 mm) biopsies had similar diagnostic concordance rates when compared to the final pathology from ESD [53]. Among those with early gastric cancer likely amenable to endoscopic treatment, if H. pylori is detected on biopsy, its eradication may also reduce the risk of metachronous gastric cancer after endoscopic resection [54]. For more advanced cancers eligible for systemic treatment, gastric cancer biopsies should be tested for human epidermal growth factor receptor 2 (HER2) positivity, as the ToGA trial demonstrated for these patients, trastuzumab with chemotherapy resulted in longer overall survival compared to chemotherapy alone [55]. Finally, after a single lesion is identified, careful inspection for synchronous abnormalities is necessary. In one study, preoperative gastroscopy performed by endoscopists with more than 10 years of experience failed to identify 15 % of synchronous multifocal gastric cancers found on surgically resected specimens, with the mean size of missed lesions (1.57 cm) significantly smaller than the detected ones (2.14 cm) [56].
The incidence of adverse events from UGI endoscopy is low, with > 50 % due to sedation and analgesia-related cardiopulmonary complications, reportedly occurring between 1/170 and 1/10,000 [57]. Mild events range from fluctuations in heart rate, blood pressure, and oxygen saturation to serious potentially life threatening aspiration pneumonia with respiratory distress. Risk factors include older age, higher American Society of Anesthesiologists (ASA) class, history of cardiopulmonary disease, prolonged procedure, and prone patient position [57]. The remaining complications relate to perforation, bleeding, and infection. Perforation rates range from 1/2500 to 1/11,000 and are most likely in patients with anatomical variants or abnormalities such as esophageal and duodenal diverticulum, esophageal strictures, or malignancies of the UGI tract [57]. Bleeding rates are likewise low, and the platelet threshold for diagnostic and therapeutic upper endoscopies are > 20,000/mL and > 50,000/mL, respectively. Preoperative management of antiplatelets and anticoagulants depends on their indications, and the procedure’s risk of bleeding [58]. When bleeding from friable tumor is encountered, hemostasis is often refractory to conventional endoscopic tools, though a novel inorganic proprietary agent has shown promise by causing mechanical tamponade, and activation of the clotting cascade [59]. Finally, infectious complications are either due to improper processing of endoscopy equipment or the procedure itself. Among UGI procedures relating to gastric cancer specifically, antibiotic prophylaxis is recommended when placing percutaneous endoscopic gastrostomies and jejunostomies [60].
Staging
Management decisions of gastric adenocarcinoma depend on accurate tumor staging. The TNM staging model developed by the American Joint Committee on Cancer and the International Union Against Cancer is based on the degree of tumor (T), nodal (N) involvement, and evidence of distant metastasis (M). T staging reflects depth of tumor invasion into the stomach wall. Tumor size does not play a role in T staging, however, is an important factor when deciding suitability of endoscopic treatment in cases of early gastric cancer. N staging describes the number of malignant nodes involved, whereas the location of nodal involvement was considered in earlier TNM classifications. M staging denotes presence or absence of distant metastatic disease. Preoperative clinical staging includes endoscopic ultrasound (EUS) , possibly with fine needle aspiration (FNA) , for the most accurate noninvasive locoregional T and N staging, while distant metastasis is evaluated by multidetector computed tomography (MDCT) of the chest, abdomen, and pelvis. This approach in turn risk stratifies patients to endoscopic treatment such as EMR, ESD, surgery, or systemic chemotherapy. While both the National Comprehensive Cancer Network of the United States and the European Society of Medical Oncology endorse EUS staging of nonmetastatic lesions possibly treated endoscopically, a recent Asian consensus did not include this modality for routine staging due to its T stage limitations, as discussed below [61].
Staging EUS when performed is preferably with the radial echoendoscope. The circumferential view, which is perpendicular to the shaft axis, permits assessment of wall layer involvement, abnormal lymph nodes, and tumor invasion into adjacent structures. Prior to EUS evaluation, any food contents or bubbles within the stomach should be removed or washed off, and air is suctioned from the stomach. To achieve close acoustic coupling between the echoendoscope tip and the lesion, either 300–400 cc of 0.9 % isotonic saline or water can be instilled into the stomach, or a water-filled balloon placed at the echoendoscope tip can be inflated. The endoscopist should be mindful of the risk of aspiration with the patient in the left lateral decubitus position. EUS starts by positioning the probe in the antrum, instillation of water into the stomach/insufflation of the water-filled balloon, and slow withdrawal to the esophagogastric junction. With the 7.5–12-MHz echoendoscope, the normal gastric wall is represented as a 3–4-mm five-layer structure with alternating echogenicity (Fig. 9.2). The first two layers correspond to the superficial and deep mucosal layers. The third layer which is hyperechoic reflects the submucosa, while the fourth hypoechoic layer is the muscularis propria, and the outermost 5 th hyperechoic layer represents the subserosal fat and serosa. Higher-frequency (> 12 MHz) probes will depict the gastric wall with greater resolution with nine layers, but the depth of penetration is limited, potentially affecting nodal staging. When the lesion of interest is visualized, it is important to position the tip of the echoendoscope perpendicularly to avoid inaccurate staging from tangential views. With fine movements, the scope can be advanced, withdrawn, and torqued to provide a comprehensive assessment. Clinical T staging by EUS is categorized as :
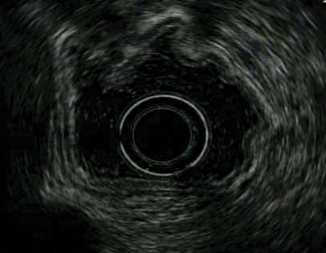

Fig. 9.2
Normal gastric wall represented as a 3–4-mm five-layer structure with alternating echogenicity
T1a
Tumor limited to the mucosa (first or second layer)
T1b
Tumor limited to the submucosa (third layer). The outer margin of the hyperechoic third layer is smooth (Fig. 9.3)
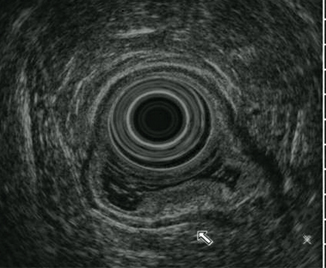

Fig. 9.3
T1b tumor limited to the submucosa ( third layer)
T2
Tumor extends into but not through the muscularis propria (fourth layer). The outer margin of the hypoechoic fourth layer is intact (Fig. 9.4)
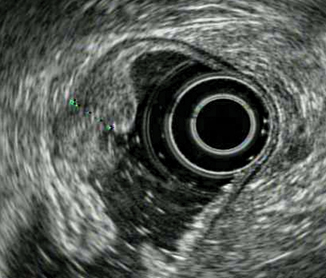

Fig. 9.4
T2 tumor extending into but not through the muscularis propria ( fourth layer)
T3
Tumor penetrates the subserosa (fifth layer) (Fig. 9.5)
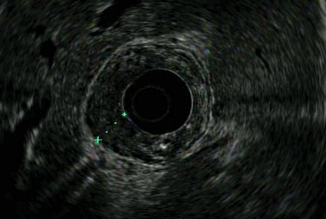

Fig. 9.5
T3 tumor penetrating subserosal connective tissue without invasion of visceral peritoneum or adjacent structures
T4
Tumor invades into adjacent vascular structures (aorta or celiac axis) or organs (liver, pancreas, spleen) (Fig. 9.6)
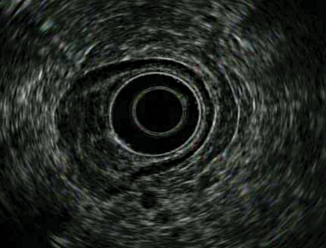

Fig. 9.6
T4a showing tumor invading serosa (visceral peritoneum)
Instead of a discrete mass, gastric cancer can alternatively present as linitis plastica from diffuse tumor infiltration causing a rigid stomach that does not insufflate well with air. On EUS, there is a markedly thickened gastric wall with loss of the normal five-layer pattern (Fig. 9.7).
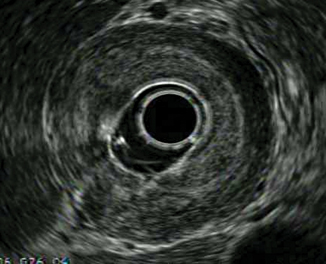

Fig. 9.7
Linitis plastica, represented on EUS as a markedly thickened gastric wall with loss of normal five-layer pattern
Once the primary tumor has been T staged, perigastric and regional lymph nodes such as gastrohepatic ligament and celiac axis nodes are assessed. EUS features suggestive of malignant lymph nodes include size greater (vs less) than 1 cm, circular (vs elliptical) shaped, sharp (vs irregular) margins, and hypoechogenicity (vs others). As previously noted, it is the number, not the location or proximity to the primary lesion, which dictate N staging. Cardoso et al. conducted a systematic review and meta-analysis of 22 studies between 1998 and 2009, reporting a pooled accuracy for T staging of 75 % with a moderate Kappa of 0.52. EUS T staging was more accurate for T3 and T4 disease, than T1 and T2 disease [62]. Understaging can be due to microscopic infiltration, while peritumoral inflammation may result in overstaging. EUS pooled accuracy for N staging was 64 %, with 74 % sensitivity and 80 % specificity [62]. An earlier meta-analysis likewise noted greater T stage accuracy for more advanced disease, but also demonstrated this for N staging, where the pool sensitivity of N1 disease was 58.2 %, and N2 was 64.9 % [63]. In comparison to other cross sectional imaging modalities, a systematic review reported the diagnostic accuracy of T staging from EUS (65–92 %) was comparable to MDCT (77–88 %) and magnetic resonance imaging (MRI) (71–82 %) [64]. These three modalities also had similar sensitivities for N staging of between 68 % for MRI to 71 % for EUS and 80 % for MDCT [64]. Site of disease can affect accuracy of T staging, and lesions at the cardia, lesser curvature along the upper body, and the incisura can be challenging to visualize. Further, tumor size greater than 3 cm has been associated with overstaging, while undifferentiated histology correlated to understaging in a retrospective Korean study comparing EUS T staging accuracy with EMR histology [65]. Limitation of EUS for nodal staging is primarily due to the inability to differentiate benign reactive from malignant lymph nodes. The previously described EUS criteria for malignant lymph nodes are found infrequently together. Individually, these features are not specific for cancer involvement. However when all features are present, this can confer an 80 % chance of malignancy infiltrating the lymph node [66]. The likelihood of lymph node metastasis also increases with T stage [67]. Lymph nodes beyond the depth of penetration of the echoendoscope will not be detected, and this occurs more commonly for those along the greater curvature than the lesser curvature. EUS’s ability to M stage is limited to assessing for disease in the left lobe of the liver, the left adrenal gland, the presence of ascites or pleural effusion, and mediastinal lymphadenopathy. EUS may detect radiographically occult liver metastases, though this is uncommon [68]. For the detection of ascites, EUS has been reported to be more sensitive than either laparoscopy/laparotomy or combined CT and ultrasound in an Asian study [69]. Found between the echoendoscope tip and external to the gastrointestinal tract and visceral organs like the liver, ascites is usually seen as a triangular anechoic space that can change shape with patient position. Finally, EUS guided FNA with a linear echoendoscope has successfully diagnosed malignant ascites, though a negative ascites fluid cytology does not exclude peritoneal carcinomatosis [70]. Endoscopists should be mindful that traversing of the EUS needle across the tumor into ascites fluid can result in false positive cytology, and seeding of the peritoneal cavity.
The type of complications associated with EUS and FNA are similar to UGI endoscopy, and include cardiopulmonary events from sedation and analgesia, perforation, bleeding, and infection [71]. In a systematic review of EUS-FNA studies mostly of pancreatic lesions, perforation, hemorrhage, infections, and post-EUS-FNA pancreatitis were reported to be 0.02 % (2/10,941), 0.13 % (14/10,941), 0.05 % (5/10,941), and 0.44 % (36/8246), respectively [72]. To minimize risk of perforation, endoscopists should be particularly cognizant of the semi-blind nature of the cervical intubation, and the rigidity of the echoendoscope tip compared to a standard UGI endoscope. Similar to upper endoscopy, preoperative management of antiplatelets and anticoagulants depend on their indications compared to the procedural risk of bleeding.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







