(1)
Department of Surgical Oncology, Division of Surgery, The University of Texas MD Anderson Cancer Center, 1400 Pressler St., Unite #1484, 77030-4009 Houston, TX, USA
(2)
Department of Surgical Oncology, Division of Surgery, Infusion Therapy and Mobile Procedure Team, The University of Texas MD Anderson Cancer Center, 1400 Pressler St., Unite #1484, 77030-4009 Houston, TX, USA
Keywords
PostgastrectomyComplicationsMorbidityMortalityGastric cancerIntroduction
Gastric surgery is complex and is associated with high rates of morbidity, mortality, and hospital readmission. Complications can be divided into early (most often defined as within 30 days of surgery) and late. The majority of early complications after gastric surgery include common surgical morbidities such as anastomotic leakage, wound infections, abscess, bowel obstruction, and the risks of general surgery, such as cardiovascular events, respiratory complications, and venous thrombosis. Postoperative complications can lead to readmission, which is of concern because of the proposed reductions in payments by the Centers for Medicare and Medicaid Services to hospitals with high readmission rates. Readmission rates after gastrectomy are significant, ranging from 10 to 20 % within 30 days of surgery. Late complications include not only delayed presentation of early complications but also unique complications of gastrectomy, including the so-called postgastrectomy syndromes. Postgastrectomy syndromes include bile reflux gastritis, dumping syndrome, afferent and efferent limb syndrome, Roux stasis syndrome, and postvagotomy diarrhea. This chapter discusses overall morbidity and mortality rates of patients who have undergone resection for gastric cancer and will describe early and late complications of gastric surgery, with a focus on diagnosis, medical management, and surgical treatment.
Early Postoperative Complications
Overall Morbidity and Mortality Rates
Clinical trials often provide some of the highest quality data on morbidity and mortality rates for surgery. Several prospective trials in gastric cancer contain early postoperative outcomes and have reported fairly consistent morbidity and mortality rates. Many of these randomized trials compared regional and extended lymphadenectomy, although some studies regarded resection of the spleen and the tail of the pancreas as necessary for removing the D2 lymph nodes. The Dutch trial of 711 patients undergoing resection with curative intent reported morbidity and mortality rates of 25 and 4 %, respectively, in patients undergoing D1 dissection and 43 and 10 % in patients undergoing D2 dissection [1]. The Medical Research Council Gastric Cancer Surgical Trial, in a similar study to the Dutch trial, compared early complications after D1 dissection with those after D2 dissection. Morbidity and mortality rates in the D1 dissection group were 28 and 7 %, respectively, compared with morbidity and mortality rates of 46 and 13 % in the D2 dissection group [2]. The Italian Gastric Cancer Study Group, in a prospective multicenter trial evaluating complications after pancreas-preserving D2 lymph node dissection, reported a postoperative morbidity rate of 21 % and a hospital mortality rate of 3 % [3]. Important findings from this study, in addition to the low overall morbidity rate, were variable hospital mortality rates of 1 % after subtotal gastrectomy and 7 % after total gastrectomy. The Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial of perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer included postoperative complications as outcome measures. Morbidity and mortality rates for patients in this trial were 45 and 6 %, respectively, and these rates were similar in patients treated with chemotherapy or with surgery alone [4]. Table 17.1 summarizes the overall complication rates for gastrectomy in gastric cancer trials and provides an overview to guide more in-depth discussion of specific complications. In addition, a recent Cochrane review has summarized four randomized clinical trials evaluating the need for abdominal drainage after gastrectomy. This review reported a low 30-day overall mortality rate (1.4 %), with no difference in the rate of complications between patients with or without drain placement at surgery [5].
Table 17.1
Postoperative morbidity and mortality in clinical trials of surgery for gastric cancer
Trial, year | Morbidity rate (%) | Mortality rate (%) | Anastomotic leakage | Reoperation rate | Length of stay (days) |
|---|---|---|---|---|---|
MRC Gastric Cancer Surgical Trial, 19962 | 28–46 | 7–13 | 11–26 % | ND | 14 |
Italian Gastric Cancer Study Group, 19983 | 21 | 3 | 7 % | 3 % | 17 |
Dutch Trial, 19991 | 25–43 | 4–10 | ND | ND | 14–16 |
MAGIC Trial, 20064 | 45 | 6 | ND | ND | 13 |
Programmatic databases such as The American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database and The Veterans Affairs National Surgical Quality Improvement Program (VA NSQIP) can also provide high-quality data on 30-day morbidity and mortality after gastrectomy for cancer. In a recent study that used the ACS NSQIP participant use file from 2005 through 2010, overall postgastrectomy serious morbidity occurred in 24 % of patients and the 30-day mortality rate was 4 % [6]. Patients undergoing total gastrectomy had serious morbidity and mortality rates of 29 and 5 %, respectively, and patients undergoing partial gastrectomy had serious morbidity and mortality rates of 20 and 3 %, respectively (Table 17.2). An older VA NSQIP study of patients undergoing gastrectomy for cancer from 1991 through 1998 reported 30-day morbidity and mortality rates of 33 and 8 %, respectively [7].
Table 17.2
Postoperative 30-day morbidity and mortality rates in patients undergoing total and partial gastrectomy for gastric cancer in ACS NSQIP from 2005 to 2010 [6]
Operation | Serious morbidity (%) | Mortality (%) | Sepsis (%) | Organ space infection (%) | Reoperation rate (%) | Median length of stay (days) |
|---|---|---|---|---|---|---|
All gastrectomy | 24 | 5 | 7 | 7 | 8 | 12 |
Partial gastrectomy | 20 | 3 | 6 | 6 | 6 | 12 |
Total gastrectomy | 29 | 5 | 9 | 9 | 10 | 13 |
Surgical Site Infections
Superficial, deep, and organ space infections occur in approximately 6, 1, and 7 %, respectively, of patients undergoing gastrectomy for cancer, with only slightly higher rates of organ space infection in patients undergoing total gastrectomy than in those undergoing subtotal gastrectomy [6].
Wound Disruption
Wound dehiscence occurs in 1–2 % of patients.
Pulmonary Complications
Postoperative pneumonia occurs in about 7 % of patients but can be as high as 12 % in high-risk populations, such as patients receiving care in the United States Department of Veterans Affairs hospital system [6, 7]. Failure to wean from ventilator assistance after 48 h and reintubation occur in 6 % of patients, with higher rates in patients undergoing total gastrectomy.
Bleeding
Postoperative hemorrhage or requirement of transfusion of more than 4 units of blood occur in approximately 3 % of patients [7].
Cardiac Complications
Cardiac arrest and myocardial infarction occur in 1–3 % of patients.
Deep Vein Thrombosis and Pulmonary Embolism
Venous thrombosis occurs in 1–2 % of patients. Pulmonary embolism also occurs in 1 % of patients undergoing partial gastrectomy and in 2 % of patients undergoing total gastrectomy [6].
Urinary Tract Infection
Urinary tract infection occurs in up to 6 % of patients.
Reoperation
Reoperation is needed and occurs in 6–10 % of patients and occurs more often in patients undergoing total gastrectomy.
Delayed Gastric Emptying
Early delayed gastric emptying after subtotal gastrectomy is not infrequent and is likely exacerbated by regional lymph node dissection along the lesser curvature of the stomach with resection of the vagus nerve branches. Medical management includes supplementing the patient’s nutrition through total parenteral nutrition or tube feedings and prescribing promotility agents such as metoclopramide and erythromycin. Surveillance endoscopy findings from patients after overnight fasting have shown food retention in 14–38 % of patients after subtotal gastrectomy , but this finding is often not associated with symptoms of delayed gastric emptying [8].
Anastomotic Leaks
Anastomotic leakage occurs in 5–10 % of patients undergoing total gastrectomy for gastric cancer [2, 9, 10]. The majority of leaks can be treated conservatively without reoperation. Anastomotic leakage significantly increases mortality and also appears to be associated with poor oncologic prognosis [10, 11]. In a large series of over 1000 total gastrectomies performed over a 30-year period, the associated mortality rates were 19 % in patients with anastomotic leakage treated without surgery and 64 % in patients treated with surgery [9]. The increased mortality rates associated with reoperative surgery have led to efforts to manage leaks nonoperatively. Endoscopic stent placement has been shown to be a safe procedure to manage anastomotic leaks conservatively after gastrectomy and esophagectomy. Although most series are small, successful healing rates for patients with an anastomotic leak treated with stent placement range from 75 to 90 % [12, 13].
Duodenal Stump Leak
The duodenal stump is a relatively infrequent site of leakage after subtotal or total gastrectomy, with reported frequency rates of 2–3 % [3, 14, 15]. Leakage from the duodenal stump with resultant inflammation has also been reported as a negative prognostic factor for overall survival after surgery for gastric cancer [14]. Management may be nonoperative with percutaneous drain placement or may require reoperation with drain placement, although further attempts at duodenal stump closure are rarely successful. Figure 17.1 shows a computed tomography (CT) image of a patient with a duodenal stump leak after subtotal gastrectomy.
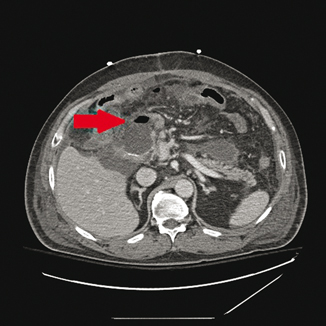
Fig. 17.1
Computed tomography (CT) image of a patient with duodenal stump leakage after subtotal gastrectomy. Red arrow indicates fluid and air at duodenal stump staple line
Late Postoperative Complications
Dumping Syndrome
Dumping syndrome can be classified on the basis of time ( early versus late) and symptom type ( vasomotor versus gastrointestinal). Early dumping typically occurs within 30 min of eating, whereas late dumping occurs several hours after eating. Early dumping syndrome is likely the result of the rapid emptying of hyperosmolar food into the small bowel. Owing to the rapid hyperosmolar load into the bowel, fluid shifts into the bowel and causes a sympathetic response [16]. Excessive gastrointestinal hormone secretion into the bowel and local peristaltic responses also play a role in this syndrome. Early dumping syndrome symptoms often include nausea, abdominal cramping, diarrhea, tachycardia, and possibly hypotension. Late dumping is often attributed to the aggressive insulin response to hyperglycemia induced by the rapid emptying of carbohydrate-rich food into the small bowel and occurs within 2–3 h after a meal. The insulin response leads to subsequent hypoglycemia, with symptoms such as fatigue, weakness, and diaphoresis. Vasomotor predominant dumping includes flushing, diaphoresis, palpitations, and tachycardia. Gastrointestinal predominant dumping includes symptoms of nausea, emesis, abdominal pain and cramping, and diarrhea.
Diagnosis
As dumping is a relatively frequent postgastrectomy issue, diagnosis is made predominantly based on the presence of typical symptoms and inciting factors. In a large survey of over 1000 gastrectomy patients, 68 % of patients had early dumping syndrome, and 38 % had late dumping syndrome [17]. Although criteria for dumping syndrome have been developed on the basis of a 50-g glucose provocative test, subjective symptoms remain the mainstay of diagnosis [18]. Diagnosis can also be confirmed with improvement from dietary modifications. Patients with early dumping syndrome are susceptible to developing late dumping syndrome. Patients who lose more weight after surgery are also prone to dumping syndrome [17].
Medical Management
Dietary management is the primary treatment modality for dumping syndrome and is often successful. Patients should avoid foods with high levels of simple carbohydrates (sugar) and attempt to eat small, frequent meals with foods high in fiber and protein. Patients should eat 6–8 meals per day and restrict fluids while eating. Vasomotor-predominant dumping symptoms can be improved if the patient rests in the supine position for 20–30 min after eating. Severe dumping syndrome refractory to standard dietary changes may be improved with octreotide [19, 20]. Long-acting repeatable (LAR) intramuscular octreotide injection appears to be as effective at improving postoperative dumping as short-acting subcutaneous octreotide. The LAR form of octreotide has the obvious benefit of monthly injection compared with three injections per day of the short-acting formulation. Comparison studies have shown that the LAR form of octreotide improves quality of life scores [20]. Limitations to octreotide therapy include an apparent tolerance to the therapeutic effect and long-term side effects that include gallstones, diarrhea, and steatorrhea [21, 22].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







