Samir V. Parikh, Nabil J. Haddad, Lee A. Hebert
Retarding Progression of Kidney Disease
Progression to end-stage renal disease (ESRD) usually occurs because of activity of the primary kidney disease and the mechanisms of natural progression (see Chapter 79). In those older than 40 years of age, the nephropathy of aging can also contribute to progression. Its pathogenesis is unclear; however, the nephropathy of aging appears to be categorically different from that of natural progression because, unlike natural progression, the nephropathy of aging proceeds without important increases in proteinuria (see Chapter 67). Whether therapy can slow the nephropathy of aging is unclear. However, natural progression is treatable. This chapter focuses on its treatments.
Natural progression creates a vicious cycle that is approached when nephron loss leads to hyperperfusion of the surviving glomeruli and to the metabolic dysfunctions of decreased glomerular filtration rate (GFR). The vicious cycle is entered when the hyperperfusion and metabolic dysfunctions are sufficient to inflict kidney injury. At this point, nephron loss begets more nephron loss. Described herein are evidence-based therapies to prevent entry into the vicious cycle or to slow the cycle once it has been entered.
Level of Glomerular Filtration Rate and the Risk of Natural Progression
Typically, natural progression does not proceed until nephron loss exceeds 50%.1,2 For example, unilateral nephrectomy as occurs in living kidney donors does not usually lead to natural progression.1,2 However, a normal solitary kidney is vulnerable to natural progression if the condition is congenital or acquired early in life3 or if it is associated with obesity, hypertension, hyperlipidemia, or hyperglycemia. Low birth weight, particularly in males, may lead to ESRD in adulthood, by natural progression, presumably because of defective nephron development.2
Proteinuria Magnitude and the Risk of Natural Progression
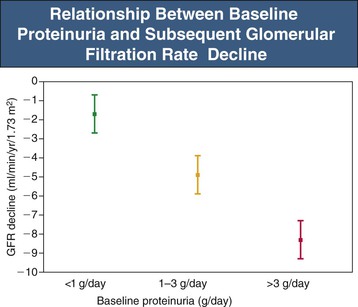
Proteinuria-induced glomerular and renal tubular injury is a key mechanism of natural progression (see Chapter 79). The threshold for natural progression attributable to proteinuria appears to be crossed when proteinuria exceeds 500 mg/day.2 Proteinuria magnitude is the strongest single risk factor in chronic kidney disease (CKD) progression (Fig. 80-1).1 An exception is highly selective proteinuria (the urine protein is almost entirely albumin), which can persist in the nephrotic range for more than 10 years without causing renal structural damage.2
Diagnosis of Natural Progression
Natural progression is a diagnosis of exclusion. Therefore, in the CKD patient showing signs of progression, it must first be determined whether the primary renal disease is active. If that can be reasonably excluded, it must then be determined whether another kidney disorder has been superimposed (see Chapter 71). The nephropathy of aging must also be excluded. This is relatively easy because it starts after 40 years of age, the decline in GFR is slow (about 1 ml/yr), and it generally does not involve important increases in proteinuria (see Chapter 67).1 Findings that help confirm the diagnosis of natural progression are as follows:
▪ The serum creatinine (SCr) generally is above the expected level for the patient’s age, sex, and race (Box 80-1). Natural progression is unlikely if the SCr is normal.
▪ Natural progression is usually indolent—for example, the increased SCr has been stable for at least several years before signs of progression occur.4 An exception can occur when there are extraordinarily strong forces promoting natural progression, such as a very high salt intake, poorly controlled hypertension, or both.4
▪ Increase in proteinuria is usually the first sign of natural progression. Only after a year or more of rising proteinuria does the SCr begin to increase.4
▪ The urine sediment is unremarkable, although broad hyaline and granular casts may be present.
In summary, natural progression is seen mainly in those with increased SCr. Increasing proteinuria is the first sign of natural progression. Only after a prolonged period of increasing proteinuria does the SCr begin to increase. This pattern is also commonly seen in CKD patients whose condition is progressing because of activity of the glomerular disease. In contrast, certain forms of CKD can show substantial progression even though proteinuria is minimal (Box 80-2).
Monitoring Kidney Disease Progression
Progression can be monitored by structural changes—for example, with renal biopsy or renal ultrasound—or by functional changes—for example, change in GFR or proteinuria. Here we discuss the latter, which are the most practical and commonly used methods to monitor progression.
Monitoring Proteinuria Trends
Proteinuria is the strongest single predictor of GFR decline. Therapies that decrease proteinuria generally slow GFR decline (see Chapter 79).1,5 The most widely used methods to assess proteinuria are measurement of urine albumin and urine total protein (see Chapter 4). The latter is the sum of urine albumin plus nonalbumin proteins. In the typical CKD patient the nonalbumin urine proteins consist mainly of low-molecular-weight proteins such as β2-microglobulin. They escape reabsorption because of tubular damage (see Chapter 15). In this chapter, total proteinuria is referred to simply as proteinuria.
Recently, albuminuria measurement has gained favor over proteinuria measurement in CKD management. However, of the two studies that compared urine albumin-creatinine ratio (ACR) and urine protein-creatinine ratio (PCR) as predictors of GFR decline, one found ACR better than PCR,6 and the other found PCR not inferior to ACR.7 Kidney Disease: Improving Global Outcomes (KDIGO) recommends ACR. However, albumin assays are expensive compared with protein assays. Furthermore, above a proteinuria of 500 mg/day, ACR and PCR generally change in parallel.7,8 If 24-hour proteinuria is below 500 mg/day, this parallelism is lost and ACR is more sensitive. On this basis, we recommend ACR for detection of early CKD progression. Thereafter, PCR is recommended. The recommended methods for monitoring proteinuria trends in individual patients are shown in Table 80-1. The key points are as follows:
▪ The gold standard for monitoring proteinuria is the protein content of an accurately collected 24-hour urine specimen. Unfortunately, in practice, nominal 24-hour urine collections often are largely undercollections or overcollections. For this reason we prefer the term “intended” 24-hour urine collection when such collections are made.9,10
▪ Creatinine should be measured in all intended 24-hour urine collection specimens. The PCR of the intended collection is a reliable estimate of the PCR of the complete 24-hour collection, if the intended 24-hour urine collection is at least 50% complete based on its total creatinine content.9,10
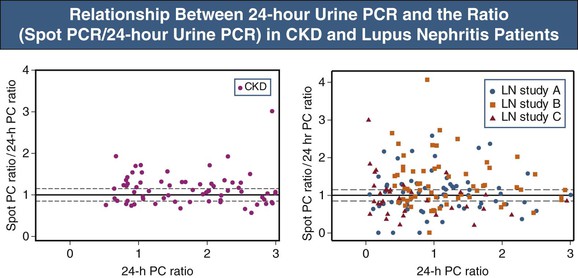
▪ In individual patients, the PCR of spot (single void) urine collections is an unreliable estimate of proteinuria magnitude given the large and inherent variability of the PCR (Fig. 80-2).
▪ The inconvenience of 24-hour urine collections can be lessened by the following:
Table 80-1
Recommended and nonrecommended methods for monitoring proteinuria or albuminuria.
E = (140 − Age) × Weight (nonobese) in kg × 0.2 × 0.85 if female.
| Recommended and Nonrecommended Methods for Monitoring Proteinuria or Albuminuria | |
| Methods | Comments |
| Recommended | |
| PCR or ACR* of intended 24-h urine collections that are at least 50% complete based on creatinine content† | Most accurate method (see text). Can also be used to assess nutrient intake relevant to CKD management (e.g., Na, K, urea nitrogen, water). Inconvenient, but this can be lessened. See text. |
| PCR or ACR ratio from overnight collections (first morning void) | More convenient than 24-h collections but provides a lower estimate of proteinuria than 24-h collections and is more vulnerable to artifacts (see text). |
| Not Recommended | |
| Intended 24-h urine collection in which protein or albumin is measured but creatinine is not measured | Large overcollections and undercollections are common in intended 24-h urine collections. If creatinine content is not measured, the degree of overcollection or undercollection cannot be reliably assessed. |
| Spot PCR or ACR | Convenient but highly inaccurate estimate. Cost-benefit ratio is low because cost is about the same as 24-h urine testing, but often the results of spot testing are misleading. |
| Dipstick test for proteinuria (albuminuria) | Convenient and low-cost point-of-care measure but not reliable for monitoring CKD patients. |
* ACR is recommended if 24-hour proteinuria is below 500 mg (PCR if below 0.3 for average-sized person). PCR is recommended if 24-hour proteinuria is above 500 mg.
† Degree of completeness of an intended 24-hour urine collection = measured creatinine content (M)/expected creatinine content (E).
An M/E ratio exceeding 0.5 is evidence that the collection is more than 50% complete.
ACR, Albumin-creatinine ratio; CKD, chronic kidney disease; PCR, protein-creatinine ratio.
Dipstick testing for proteinuria is convenient but unreliable (see Chapter 4). It is not recommended for assessing proteinuria trends in individual CKD patients.9
Proteinuria testing in CKD is recommended every 6 to 12 months if proteinuria is low level (e.g., <500 mg/day, ACR <0.2, PCR <0.3 when expressed as mg/mg creatinine, in an average-sized person) and every 2 to 4 months for those with heavier proteinuria. If PCR increases from below 0.5 to 1.0 or higher, or from below 1.0 to 2.0 or hither, likely these are real changes.11 Most of the measures that slow natural progression also reduce proteinuria.
Monitoring Glomerular Filtration Rate Trends
In individual patients, it is usually sufficient to monitor GFR trends by serial SCr measurements. In interpreting SCr change, one must keep in mind the circumstances that can change SCr by mechanisms that do not involve a change in GFR, as follows: increased creatinine production (eating cooked meat, creatine ingestion, increased exercise, increasing muscle mass, fenofibrate therapy), or decreased creatinine production (vegetarian diet, muscle wasting, decreased exercise). Also, SCr can be increased spuriously by certain drugs and by increased serum ketones (from fasting or poor diabetes control) or actually increased by drugs that decrease tubular secretion of creatinine (cimetidine, trimethoprim). If a chronic progressive change in creatinine production is occurring (e.g., muscle wasting), GFR trends can be monitored by measuring serial 24-hour urine creatinine clearance. Creatinine clearance is not importantly affected by change in creatinine production. If creatinine clearance is used, an accurate 24-hour collection is essential.1
Glomerular filtration rate can also be monitored by the creatinine-based estimated glomerular filtration rate (eGFR) estimating equations—in particular, the Modification of Diet in Renal Disease (MDRD4) and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations (see Chapter 3). These approaches to standardizing GFR assessment have greatly advanced the study of CKD epidemiology. In addition, they account for the influence of age on GFR. This is important in monitoring GFR trends over 10 years or more.1 However, the eGFR equations have important limitations when applied to individuals, as follows:
▪ The MDRD4 and CKD-EPI equations assume that all patients of the same age, sex, and race have the same rate of creatinine production and the same body surface area (BSA). As a result, these equations substantially underestimate actual GFR in those with high creatinine production and substantially overestimate actual GFR in those with low creatinine production.12
▪ Because of inaccuracy, clinical laboratories generally do not report eGFR by MDRD4 or CKD-EPI equation if the eGFR is greater than 60 ml/min/1.73 m2. In this circumstance the clinician must determine whether the SCr is likely to be normal or abnormal. The following can be helpful:
• Large normal persons have higher SCr values than small normal persons.
• Men have higher SCr values than women.
Box 80-1 provides specific numerical recommendations for interpretation of SCr when the eGFR is greater than 60 ml/min/1.73 m2.
Glomerular Filtration Rate Trajectories in Chronic Kidney Disease
The chronic kidney disease GFR trajectory generally is well described as a linear decline; thus GFR loss per unit of time is approximately constant.1,2 However, those of African ancestry deemed to have hypertensive nephrosclerosis can experience large unexplained changes in eGFR trends, especially decreases followed by stability. A concern is that increased variability of GFR decline over time is associated with increased mortality.13
Indexing Proteinuria and Albuminuria by Estimated Glomerular Filtration Rate to Predict Risk of Chronic Kidney Disease Progression
Recent work has emphasized that at any given level of albuminuria, the lower the eGFR, the greater the risk of CKD progression. This is referred to as indexing the risk of progression according to both proteinuria and eGFR.14 Indexed GFR and albuminuria levels also are associated with increased cardiovascular mortality.
Therapy for Natural Progression
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree