Mark S. Segal, Xueqing Yu
Herbal and Over-the-Counter Medicines and the Kidney
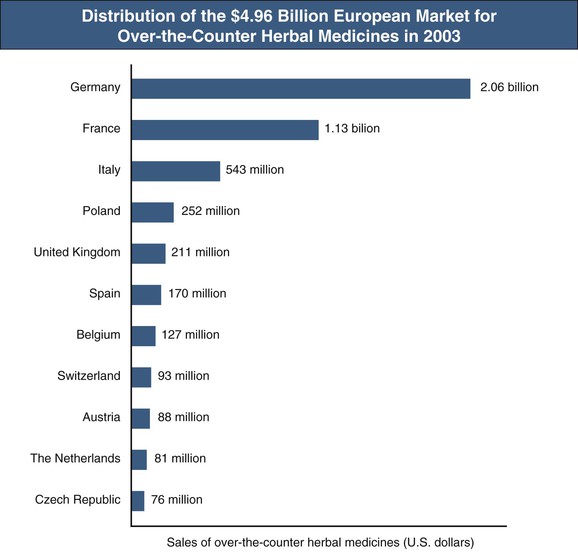
In recent years, the use of herbal medicines and dietary supplements to promote the health and to treat various chronic diseases has increased globally. An estimated one third of adults in developed countries and more than 80% of the population in many developing countries are using herbal and folk medicines to promote health and to manage common maladies, such as colds, hay fever, inflammation, heart disease, indigestion, constipation, liver cirrhosis, cancer, acquired immunodeficiency syndrome, diabetes, and central nervous system diseases.1 In Africa, up to 80% of the population depends on traditional medicine for primary health care; in China, herbal preparations account for up to 50% of the total consumption of pharmaceutical agents. There has been a surge in the popularity of herbal medicine in the West. For instance, the size of the U.S. herbal medicine market increased from $1.6 billion in 1994 to $5.3 billion in 2011.1,2 In addition, European countries spent almost $5 billion (at manufacturers’ prices to wholesalers) on over-the-counter (OTC) herbal medicines in 2003. Germany and France are the leaders in OTC sales and also have large markets for prescription herbal preparations (Fig. 78-1).3 The European Union has taken steps with the Traditional Herbal Medicinal Products Directive (THMPD, also known as Directive 2004/24/EC) implemented in May 2011 to regulate herbal remedies. THMPD mandates that all herbal medicinal products be required to obtain an authorization to market within the European Union and that herbal medicines be manufactured under Good Manufacturing Practice. Complementary and alternative medicines, including Chinese herbal medicines and herbal plants, have been used by about 50% of Australians.1
Herbal medicine was widely recorded in ancient literature. To date, there are at least 11,000 species of plants for medicinal use, and about 500 of them are commonly used by various ethnic groups.1,4 These herbal plants may be used either in their primary forms or in mixtures. However, the source and composition of botanical medicines vary depending on the prevalent local practices. Notably, these herbal remedies have not been tested for efficacy and safety, their ingredients are mostly unknown, and the dosage and route of administration are not standardized. Although the prevalence of toxicity of organs caused by traditional medicines is directly related to a combination of ignorance, poverty, lack of medical facilities, lack of legislation, and widespread belief in indigenous systems of medicine in rural areas, it has been realized recently that the problems and concerns regarding herbal medicines arise as a result of intrinsic toxicity, adulteration, contamination, substitution, misidentification, mistaken labeling, unfavorable herb-drug interactions, lack of standardization, or poor quality control.1,4 Increasing evidence for both adverse drug reactions and poisoning events associated with the use of herbal medicines has been reported worldwide. Herbal medicines have been found to be adulterated with synthetic drugs and other potentially toxic compounds. On the other hand, coadministration of herbal medicines with conventional drugs raises the potential for herb-drug interactions, which may cause altered drug elimination, undertreatment, and/or toxicity.1,4,5
Herbal Medications and the Kidney
Kidneys are particularly vulnerable to toxic injury because of their high blood flow rate, large endothelial surface area, high metabolic activity, active uptake by tubular cells, medullary interstitial concentration, and low urine pH. Renal tubules are involved in active transport and urinary concentration, and therefore the local concentration of these toxins is potentially high, leading to direct injury to tubular cells. Herbal medicines may be toxic via one or more common pathogenic mechanisms. These include alterations in intraglomerular hemodynamics, tubular cell toxicity, inflammation, crystal nephropathy, rhabdomyolysis, and thrombotic microangiopathy. Drug-related nephrotoxicity is becoming more common; people are more commonly requiring multiple medications for multiple comorbidities, and are being exposed to more diagnostic and therapeutic procedures with the potential to harm kidney function. Patient-related risk factors for drug-induced nephrotoxicity include age older than 60 years, underlying renal impairment and glomerular filtration rate (GFR) less than 60 ml/min/1.73 m2, volume depletion, diabetes, heart failure, and sepsis.6 These risk factors may also make individuals susceptible to renal toxicity from herbal medicines. An overview of the potential renal side effects of herbal medicines is shown in Table 78-1.
Table 78-1
Kidney syndromes induced by herbal medicines.
AA, Aristolochic acids.
| Kidney Syndromes Induced by Herbal Medicines | |
| Hypertension | Glycyrrhiza species (Chinese herbal teas, gancao, boui-ougi-tou) |
| Ephedra species (ma huang) | |
| Acute tubular necrosis | Traditional African medicine: toxic plants (Securidaca longepedunculata, Euphoria matabelensis, Callilepis laureola, Cape aloes), or adulteration by dichromate |
| Chinese medicine: Taxus celebica | |
| Morocco: Takaout roumia (paraphenylenediamine) | |
| Acute interstitial nephritis | Peruvian medicine (Uno degatta) |
| Tung Shueh pills (adulterated by mefenamic acid) | |
| Fanconi syndrome | Chinese herbs containing AAs (Akebia species, boui-ougi-tou, Mokutsu) |
| Chinese herbs adulterated by cadmium | |
| Papillary necrosis | Chinese herbs adulterated by phenylbutazone |
| Chronic interstitial renal fibrosis | Chinese herbs or Kampo containing AAs (Aristolochia species, Akebia species, Mu Tong, Boui, Mokutsu) |
| Urinary retention | Datura species, Rhododendron molle (atropine, scopolamine) |
| Kidney stones | Ma huang (ephedrine) |
| Cranberry juice (oxalate) | |
| Urinary tract carcinoma | Chinese herbs containing AAs |
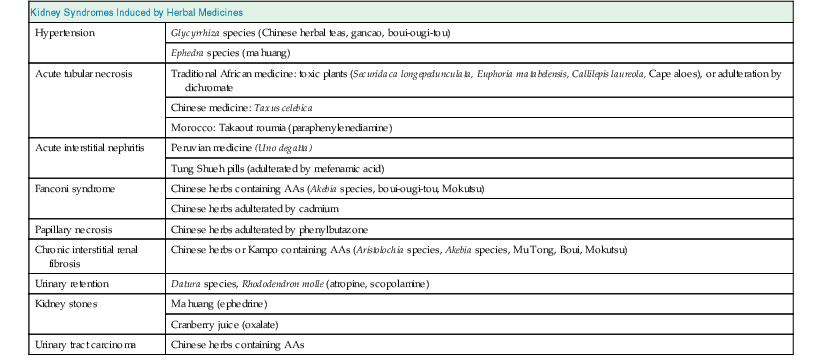
(From reference 6.)
Aristolochic Acid Nephropathy or Balkan Nephropathy
Aristolochic Acids
Aristolochic acids (AAs) are a family of structurally related nitrophenanthrene carboxylic acids that are primarily from herbal medicines such as Aristolochia species, including Aristolochia fangchi, Aristolochia clematitis, and Aristolochia manshuriensis (Table 78-2). The predominant AAs are AAI (8-methoxy-6-nitro-phenanthro-[3,4-d]-1,3-dioxolo-5-carboxylic acid) and AAII (6-nitro-phenanthro-[3,4-d]-1,3-dioxolo-5-carboxylic acid). AAs activated by human NAD(P)H:quinone oxidoreductase7 and react with DNA to form covalent dA-aristolactam and dG-aristolactam adducts.8 These aristolactam adducts are mutagenic.8 After even a single dose, AAI is detectable in the kidneys for a long period of time. Human aristolochic acid nephropathy (AAN) is reproducible in rodents in which AA intoxication results in tubular atrophy and interstitial fibrosis, leading to renal failure.9 The progressive tubular atrophy is related to impaired regeneration and apoptosis of proximal tubular epithelial cells, which is considered a possible mechanism of tubular epithelial cell deletion. The resident fibroblast activation plays a critical role in the process of renal fibrosis during AA toxicity.10 The nephrotoxic and carcinogenic effects of AAs in animals have been reported, and similar toxicities have been observed in humans. It is now known that exposure to AA causes a progressive renal interstitial fibrosis associated with urothelial malignancy.
Table 78-2
Botanicals known or suspected to contain aristolochic acid and their vernacular names.
| Botanicals Known to Contain or Suspected of Containing Aristolochic Acid | |
| Botanical Name | Common or Other Names |
| Aristolochia species | Aristolochia, Guan Mu tong, Guang Mu tong |
| Aristolochia acuminata (syn. Aristolochia tagala) | Oval leaf Dutchman’s pipe |
| Aristolochia bracteata | Ukulwe |
| Aristolochia clematitis | Birthwort |
| Aristolochia contorta | Ma Dou Ling (fruit), Bei Ma Dou Ling (root), Tian Xian Teng (herb) |
| Aristolochia cymbifera | Mil homens |
| Aristolochia debilis (syn. Aristolochia longa, Aristolochia recurvilabra, Aristolochia sinarum) | Ma Dou Ling (fruit), Tian Xian Teng (herb), Qing Mu Xiang (root), Sei-Mokkou (Japanese), Birthwort, Long birthwort, Slender Dutchman’s pipe |
| Aristolochia fangchi | Guang Fang ji (root), Fang ji, Fang chi, Makuboi (Japanese), Kou-boui (Japanese), Kwangbanggi (Korean) |
| Aristolochia heterophylla | Han Fang Ji |
| Aristolochia indica | Indian birthwort (root), Yin Du Ma Dou Ling |
| Aristolochia kaempferi (syn. Aristolochia chrysops, Aristolochia feddei, Aristolochia heterophylla, Aristolochia mollis, Aristolochia setchuenensis, Aristolochia shimadai, Aristolochia thibetica, Isotrema chrysops, Isotrema heterophylla, Isotrema lasiops) | Yellowmouth Dutchman’s pipe, Zhu Sha Lian |
| Aristolochia macrophylla (syn. Aristolochia sipho) | Dutchman’s pipe |
| Aristolochia manshuriensis (syn. Hocquartia manshuriensis, Isotrema manshuriensis) | Manchurian birthwort, Manchurian Dutchman’s pipe (stem), Guan Mutong (stem), Kan-Mokutsu (Japanese), Mokubai (Japanese), Kwangbanggi (Korean) |
| Aristolochia maxima (syn. Howardia hoffmannii) | Maxima Dutchman’s pipe, Da Ma Dou Ling |
| Aristolochia mollissima | Wooly Dutchman’s pipe, Mian Mao Ma Dou Ling |
| Aristolochia moupinensis | Moupin Dutchman’s pipe, Huai Tong |
| Aristolochia sarpentaria (syn. Aristolochia serpentaria) | Virginia snakeroot, Serpentaria, Virginia serpentary |
| Aristolochia triangularis | Triangular Dutchman’s pipe, San Jiao Ma Dou Ling |
| Aristolochia tuberosa | Tuberous Dutchman’s pipe, Kuai Jing Ma Dou Ling |
| Aristolochia tubiflora | Tubeflower, Dutchman’s pipe, Guan Hua Ma Dou Ling |
| Aristolochia versicolar | Versicoloraus Dutchman’s pipe, Bian Se Ma Dou Ling |
| Asarum canodense (syn. Asarum acuminatum, Asarum ambiguum, Asarum canadense, Asarum furcatum, Asarum medium, Asarum parvifolium, Asarum reflexum, Asarum rubrocinctum) | Wild ginger, Indian ginger, Canada snakeroot, false coltsfoot, colic root, heart snakeroot, Vermont snakeroot, Southern snakeroot, Jia Na Da Xi Xin |
| Asarum himalai(y)cum | Tanyou-saishin (Japanese) |
| Asarum splendens | Do-saishin (Japanese) |
(From reference 16.)
Aristolochic Acid Nephropathy
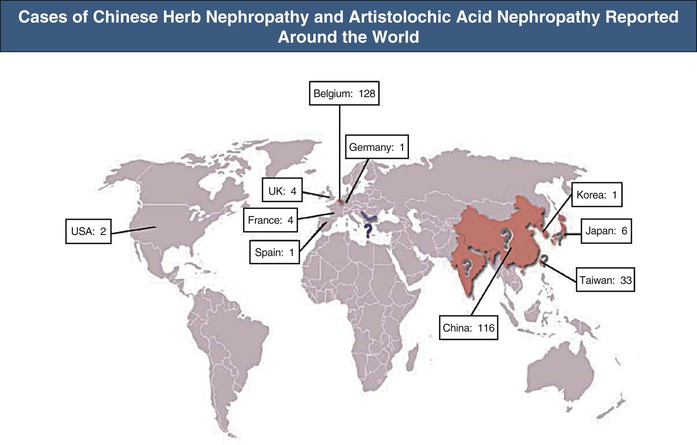
The association of kidney disease with long-term consumption of Aristolochia fangchi for slimming purposes was first reported in Belgium. More than 100 young women developed chronic kidney disease (CKD) and several developed renal and urinary tract cancer (Fig. 78-2).11 Increasing evidence demonstrates that AAs present in the herb are the compounds responsible for this renal toxicity (Figs. 78-3 and 78-4).12,13 The renal syndrome was initially referred to as Chinese herb nephropathy and later by the more specific, mechanistic name AA nephropathy. AAN is characterized by tubulointerstitial nephritis that rapidly progresses to fibrosis with deterioration of renal function, ultimately leading to end-stage renal disease (ESRD), sometimes within months after first exposure. The renal toxicity of AA is dependent on the dosage and the duration of administration.14

Epidemiology
Soon after its initial description, AAN was recognized as a global health problem.11,12,15 Since the publication of the index cases, new cases of AAN have been reported, not only in Belgium but also worldwide10,11,13 (see Fig. 78-2). The true incidence of AAN remains unknown and probably underestimated because numerous ingredients known or suspected to contain AA are used in traditional medicine in China, Japan, and India (see Fig. 78-3).16
Clinical Manifestations
The initial presentation of AAN is usually silent, and the renal disease is discovered by routine blood testing. Occasional patients present with Fanconi syndrome or with acute kidney injury (AKI) caused by acute tubular necrosis.17 Anemia is often disproportionately severe for the degree of renal impairment. The urinary sediment is unremarkable, and the urinalysis is negative for albuminuria. However, urinary excretion of low-molecular-weight proteins (e.g., β2-microglobulin, cystatin C) is markedly increased, and the ratio of urinary low-molecular-weight protein to albumin is higher than in glomerular diseases.18
Pathology
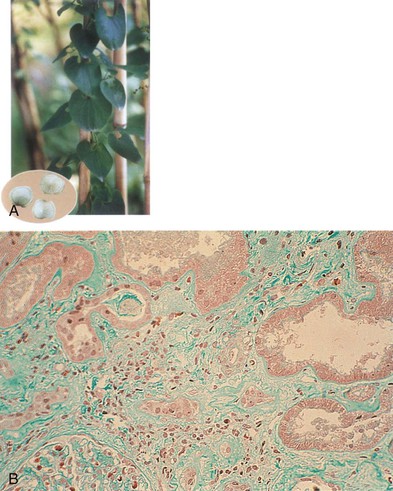
Aristolochic acid nephropathy is characterized by extensive renal interstitial fibrosis and tubular atrophy, which generally decreases in severity from the outer to the inner cortex (see Fig. 78-4). The glomeruli are relatively spared, although in the later stage of the disease there is some collapse of the capillaries and wrinkling of the basement membrane. There is endothelial cell swelling with consequent thickening of interlobular and afferent arterioles.16,19 There are no immune deposits.
Pathogenesis
The striking association between AA exposure and urothelial abnormalities was first noted in studies showing moderate atypia and atypical hyperplasia in nephroureterectomy specimens removed at the time of transplantation from individuals with AAN (see Fig. 78-4).21 The persistence of AA-DNA adducts in renal tissues of the Belgian women cohort is consistent with the postulated role of AA in urothelial cancer. Of AAN patients, 40% to 45% develop multifocal high-grade transitional cell carcinomas, mainly in the upper urinary tract.13 A case series with 15-year follow-up identified upper-tract urothelial carcinoma as a potent risk factor for the subsequent development of bladder urothelial carcinoma after kidney transplantation for AAN.22
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








