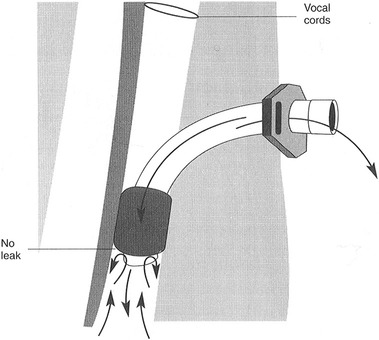
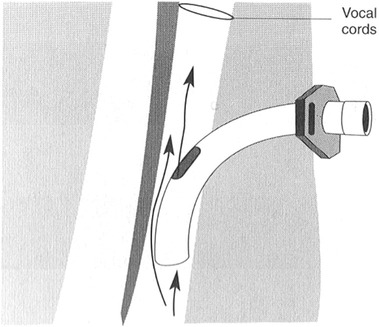
1. Detail how disorders of respiration might affect swallowing performance. 2. Discuss common medical and surgical complications that lead to or exacerbate dysphagia. 3. Review how artificial supports for respiration might interfere with swallowing. As detailed in Chapter 2, the interactions between breathing and swallowing are well known. Swallow coordination and subsequent upper airway protection depend on the normal interaction between these two related phenomena. It follows that disorders of breathing might either be the cause of dysphagia or could exacerbate it—such as after stroke.1 Some patients enter the acute hospital setting with primary respiratory tract disease, such as congestive obstructive pulmonary disease with or without accompanying dysphagia. Others enter the acute care setting for medical reasons not related to their cardiopulmonary status but have cardiopulmonary complications, such as patients who undergo cardiac bypass surgery requiring intubation, support by respirator, and/or tracheotomy. Medical and/or surgical complications that result in dysphagic complications can be classified as iatrogenic. The side effects of radiation therapy on swallowing after treatment of cancer are classified as iatrogenic but are discussed in detail in Chapter 6. Endotracheal tubes are long plastic, flexible tubes that are inserted through the mouth, through the vocal folds, and into the trachea to aid the patient in respiratory distress. They are designed to be connected to a respirator to help the patient breath. At the end of the tube is a cuff that is inflated to prevent oral secretions from entering the lungs by sealing the tracheal lumen and to keep air from escaping from the lungs past the tube (Figure 8-1). Keeping the desired respiratory volumes within the lungs is important to restore respiratory competence. Respirator settings are determined by the medical team and are implemented by the respiratory therapist. Placement of an endotracheal tube is considered a temporary measure (7 to 12 days) to establish respiratory competence. Longer periods of intubation may cause permanent laryngeal and lung injury because of local irritation to the mucosa. Granulomas and hematomas on the vocal folds and pharyngeal ulceration and edema may give rise to voice and swallowing complications. In some cases vocal fold paralysis or weakness may develop. Settings (inhalation and exhalation cycles) on the respirator are progressively adjusted to allow the patient to regain independent breathing. The endotracheal tube is removed as normal breathing patterns are achieved. Removal of the endotracheal tube does not guarantee that the patient will not experience further respiratory distress requiring reintubation. Multiple reintubations suggest that the patient’s respiratory status is tenuous and may lead to a longer term approach to airway maintenance—tracheotomy. Bypassing the functions of the upper airway with an endotracheal tube precludes the patient from eating and swallowing because the vocal folds and oral cavity and pharynx are not available for swallowing activity because of the presence of the tube. If successful extubation is achieved, the physician may request a swallowing evaluation before the patient starts oral feeding. Because the traditional method of breathing has been altered for some time, these patients may have difficulty speaking and swallowing immediately after the removal of the endotracheal tube. Partik et al.2 studied 21 patients with videofluoroscopy who were intubated for a mean of 24.6 days. They found that 86% of this group showed signs of aspiration after intubation. The majority of aspiration occurred before the swallow response, suggesting oral-stage weakness and subsequent poor laryngeal elevation. deLariminat et al.3 were interested in whether difficulty with postextubation swallowing was acute or chronic. Using swallow response times as the measurement of function, they found swallow delay on all bolus volumes on day 1; shorter, but abnormal latencies on day 2; and normal response times by day 7. These results imply that interruptions in normal respiratory function may inhibit normal swallow response time but that, over time, they will recover without specific treatment. Patients for whom weaning from endotracheal intubation is not possible may require the surgical placement of a tracheotomy tube. A vertical incision typically is made between the second and third tracheal rings so that the tube is below the level of the vocal folds to allow the medical team access to the lungs for suctioning. Other advantages over endotracheal intubation include the possibility for swallowing and speaking, less trauma to the vocal folds, and patient comfort. Tracheotomy tubes are available in various sizes, usually determined by the inner diameter of the lumen. Commonly used sizes are 8, 6, and 4 mm. The larger the tube size, generally the more difficult it is to get air around the tube up to the level of the vocal fold for phonation (Figure 8-2). Decreasing the tube size (from 8 to 6 mm) is commonly done to reinvolve the upper airway for speech and swallowing and eventually for total decannulation. However, there are no prospective, empirical data studying the role of tracheotomy downsizing and its effect on speech and swallow. Figure 8-3 shows a patient who underwent a tracheostomy with a close-up view of the tube in place. Complications of a tracheotomy tube include decreased sense of smell and taste because the direction of airflow is not through the nose and mouth, infection at the tracheotomy site, and increased secretions from the body’s response to a foreign object. Tracheomalacia, or a breakdown of tissue on the posterior pharyngeal wall as a result of constant irritation, is rare. When severe, such tissue breakdown may create a tracheoesophageal fistula with resultant aspiration of food or secretions. Tracheotomy tubes are either cuffed or noncuffed. The cuff refers to a portion on the end of the tube that can be inflated with air externally by using a syringe (Figure 8-4). When the cuff is inflated it theoretically seals off the entrance to the lungs in an effort to prevent aspiration of secretions or food. If the patient is also receiving ventilation from a respirator, the cuff ensures that the volume of air being delivered does not leak into the upper airway. The cuff may be advantageous in protecting the lungs, but its presence restricts voice and limits swallow by anchoring the larynx (Figure 8-5). In a retrospective review of videofluoroscopic evaluations of patients swallowing with the cuff inflated and deflated, Ding and Logemann4 found a higher prevalence of silent aspiration and changes in laryngeal biomechanics in the group with the cuff inflated. Reexamination of Figure 8-3 shows the external catheter line to the internal cuff on the patient’s chest. The balloon on the end of the catheter line is flat, indicating that the cuff on the end of the tracheotomy tube is deflated. When the balloon on the patient’s chest is inflated, the cuff on the end of the tracheotomy tube can be assumed to be inflated. In addition to the cuff versus no-cuff option, tracheotomy tubes may be fenestrated or nonfenestrated. A fenestration is a hole placed in the top of the tracheotomy tube to allow increased airflow to the upper airway, primarily for speaking (Figure 8-6). Numerous studies from critical care medicine have noted a higher prevalence of aspiration events in patients with tracheotomy compared with those without tracheotomy.5–7 Factors that may place patients with a tracheotomy at greater risk for aspiration include loss of subglottic air pressure,8 poor laryngeal elevation related to the mechanical presence of the tube,5 loss of upper airway sensitivity because of airway bypass, loss of the normal laryngeal closure reflex during swallow,9 and the fact that patients requiring tracheotomy are acutely ill and may not be able to coordinate a normal swallow because of muscle weakness and/or mental status fluctuations. Although there are many anecdotes that tracheotomy tethers the larynx to the neck to reduce laryngeal elevation with subsequent aspiration, only one study has measured its effects on elevation. Terk et al.10 studied seven patients with tracheotomy tubes without known dysphagia and concluded that elevation with the tracheotomy tube in place compared with when it was not in place showed no significant changes in laryngeal elevation measurements. However, factors of age, extent of respiratory illness, and prior medical history all must be considered before concluding that a tracheotomy tube does not affect laryngeal elevation. Digital occlusion in eight patients with head/neck cancer showed mixed results.11 In general, aspiration events were either reduced or eliminated. Some patients benefited with some bolus types, whereas others did not. All biomechanical measures, however, were normalized. The investigators concluded that the response to digital occlusion should be evaluated on a patient-by-patient basis. In a similar group of 16 postsurgical patients, Leder et al.12 found no difference in the prevalence of aspiration with or without finger occlusion. The use of one-way speaking valves and their effect on swallowing have been evaluated by numerous investigators with mixed findings.13–16 Comparisons of studies are difficult because of subject selection variance, type of instrumentation to measure the effects on swallowing, swallowing outcome of interest, and length of time the valve was in place before the studies were conducted to assess swallowing status. All agree, however, that the placement of a valve improves speech and reduces upper airway secretions, restores olfaction, and improves patient ability to cough and clear secretions. In a mixed group of patients with chronic respiratory disease, Leder et al.17 did not find any change in swallow-generated pharyngeal and cervical esophageal pressures with occlusion, either in those who aspirated or those who did not. Using endoscopy to study aspiration, Donzelli et al.18 studied 37 patients—first with the tracheotomy in place and then with it removed with light digital occlusion over the tracheotomy site. Although some patients showed differences in aspiration and penetration patterns, for the majority there were no significant differences. Some surgical procedures, particularly those in the neck, predispose patients to postoperative dysphagia. Dysphagia results from (1) edema that restricts movement of swallowing structures such as the pharynx; (2) interference to the peripheral nerve supply to the muscles of swallowing, such as in endarterectomy, thyroidectomy, and cervical spinal fusion; (3) loss of central nervous system (brainstem) innervation, such as from posterior fossa or skull base surgery; or (4) replacement of swallowing structures that also may interfere with peripheral cranial nerves, such as in transhiatal esophagectomy. In surgical procedures that involve the neck region, it is difficult to identify the fibers of the pharyngeal plexus that innervate the pharyngeal constrictor muscles. This surgery may result in postoperative bilateral pharyngeal weakness that cannot be explained by isolated injury to the recurrent laryngeal nerve.19 Surgical resection of all or part of the thyroid gland potentially can involve some disruption of motor and sensory branches of cranial nerve (CN) X. Unilateral vocal fold paralysis as a surgical complication compromises both voice and swallow. After follow-up of 39 patients for voice and swallow for 3 months after total thyroidectomy, Lombardi et al.20 found persistent mild symptoms of voice and swallow abnormalities. In a series of 33 patients with thyroidectomy, Wasserman et al.21 reported that 49% had preoperative swallowing difficulty, whereas 73% reported acute postoperative difficulty. They speculated that the prevalence of swallowing complaints was the result of injury to the extrinsic perithyroidal neural plexus innervating the pharyngeal and laryngeal structures. In a follow-up study of 60 patients, Pereira et al.22 found that 15% of their patients had what they termed “nonspecific upper aerodigestive” complaints, including neck strangling, voice changes, and dysphagia. None of these studies provided objective, instrumental swallowing data on the type or severity of the swallowing disorder. Ekberg et al.23 studied the swallowing ability of 12 patients before and after carotid endarterectomy. Findings for all swallowing studies were normal before surgery, but five patients had pharyngeal dysfunction and dysphagia after surgery. At 1 month after surgery, only two had swallowing complaints. These investigators speculated that the dysfunction was either attributable to peripheral nerve injury (vagus) or cerebrovascular damage during the procedure. Monini et al.24 acknowledged the potential for cranial nerve involvement after carotid endarterectomy. Their follow-up of patients included serial examinations of voice and swallow for 60 days after surgery. Although most patients had only transient difficulty, 17.5% continued to have symptoms. Of those, only 9% required rehabilitation. In a related prospective study of 19 patients after endarterectomy, swallow endoscopies were done at 5 and 90 days after surgery. During the first evaluation, 15 of the 19 patients had dysphagia. Within 1 month, 10 patients returned to their regular diet, and an additional six did so by 90 days. The investigators suggested that the swallowing skills of patients after endarterectomy be closely monitored and rehabilitation strategies implemented if difficulties persisted.25
Respiratory and Iatrogenic Disorders
BACKGROUND
ARTIFICIAL AIRWAYS
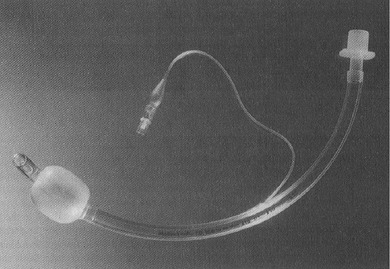
Endotracheal Tubes
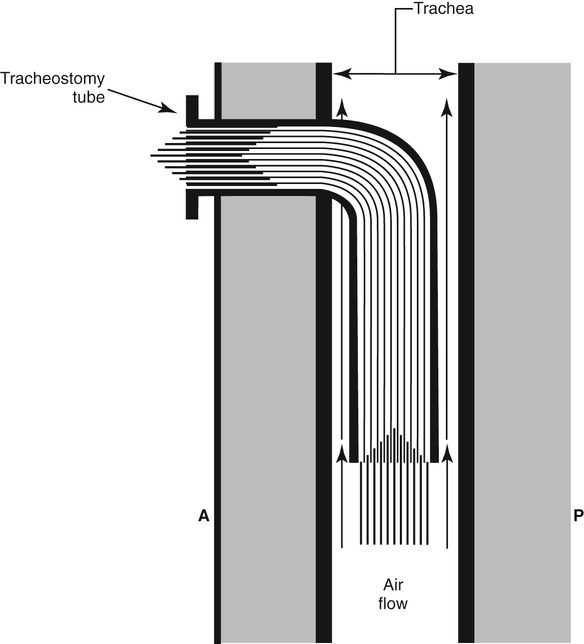
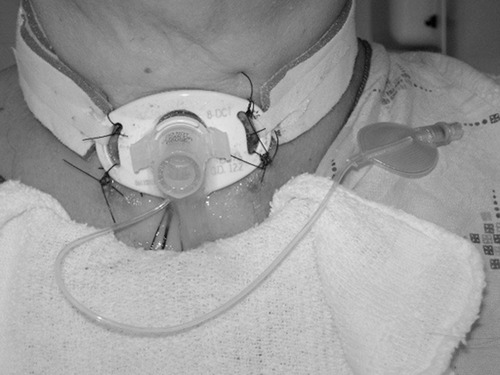
Tracheotomy Tubes
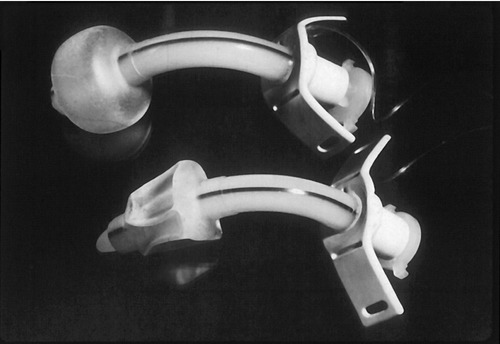
SWALLOWING AND TRACHEOSTOMY
Laryngeal Elevation
Restoring Subglottic Pressure
POSTSURGICAL CAUSES OF DYSPHAGIA
Thyroidectomy
Carotid Endarterectomy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Abdominal Key
Fastest Abdominal Insight Engine