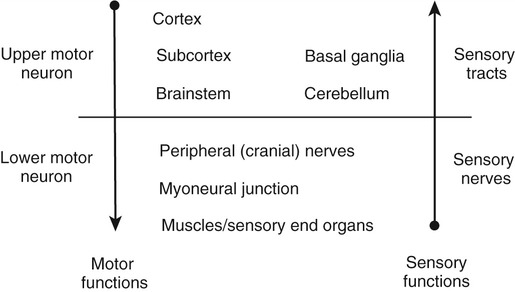
1. Explain why is it important to possess a basic understanding of the nervous system to clinically manage swallowing disorders resulting from neurologic disease. 2. Name some of the sensorimotor characteristics associated with impairments at different levels of the nervous system. 3. Identify some of the dysphagia characteristics that might be seen in diseases affecting various levels of the nervous system. 4. Describe some of the dysphagia-related problems that might be seen in patients with neurologic disease. 5. Describe some aspects of change in dysphagia over time in neurologic diseases. 6. Identify some of the more common treatment issues, decisions, options, and/or practices in different forms of neurogenic dysphagia. Swallowing disorders are symptoms of underlying disease processes. One implication of this perspective is that swallowing disorders in patients with neurologic disorders should manifest the characteristics of damage to different areas of the nervous system. This premise has long been accepted in the arena of motor speech disorders (dysarthria).1 For example, spastic dysarthria results from damage to the upper motor neuron system governing speech production. Upper motor neuron damage results in specific patterns of neuromotor impairment, including spasticity, slowed movement, exaggerated reflexes, and reduced range of movement. The characteristics of spastic dysarthria are believed to be the direct result of spasticity in the corticobulbar system governing speech production. Patients with spastic dysarthria demonstrate a slow rate of speech, limited movement of the speech articulators, equalized stress patterns, and other characteristics reflecting the underlying neuromotor characteristics of spastic weakness. A similar framework helps clinical specialists evaluate and plan treatment for patients with swallowing disorders resulting from neurologic deficit. Patients with damage to upper motor neuron systems characteristically demonstrate spastic weakness with resultant slowness and reduced range of movement. This may translate to reduced speed of swallowing (i.e., a delay in initiating one or more components of the swallow) and/or reduced range of movement in the swallowing mechanism (i.e., reduced transport of the bolus contributing to post-swallow residue). To understand better the potential clinical applications of such a framework, clinical specialists must be familiar with neuroanatomy, neurologic functions and dysfunctions of various nervous system components, and sensorimotor components of swallowing at different stages of the swallow. Chapter 2 describes the basic anatomy and neuroanatomy of swallowing functions. A summary of some common neurologic functions associated with various levels within the central nervous system follows. Motor and sensory systems work together to produce movement, including movement associated with swallowing. However, in clinical practice motor and sensory functions frequently are described separately as they may relate to impaired swallowing physiology. To facilitate a clinical perspective, a top-down approach to the nervous system is followed in which sensory and motor components are described at each level. Figure 5-1 is a simplified schematic depicting each “level” of the nervous system. Table 5-1 summarizes neurobehavioral and sensorimotor functions associated with each level. TABLE 5-1 Basic Sensorimotor Functions Associated with Different Levels of the Nervous System Before reviewing dysphagia characteristics in various cortical pathologies, a worthwhile question to ask is “Where is swallowing function represented in the human cortex?” Given the complexity of motor control involved in oropharyngeal swallowing, it is logical to implicate the frontal cortex, specifically areas involved in various components of motor control. In fact, results of both animal and human studies using lesion or cortical stimulation techniques implicate the importance of the lateral frontal cortex, the inferior frontal lobule, and the insula in various motor acts associated with feeding and swallowing. Recent studies using functional magnetic resonance imaging (fMRI) implicate a wide range of cortical, subcortical, and brainstem structures involved in swallowing performed by healthy volunteers.2 Not surprisingly, the primary motor and sensory cortical areas consistently participated in swallowing function. In a comparison of ischemic stroke patients with dysphagia and stroke patients without dysphagia, the internal capsule emerged as the only brain region significantly associated with dysphagia. However, other areas of the sensorimotor cortex and the basal ganglia also were frequently associated with the presence of dysphagia in stroke patients.3 What about cortical or hemisphere lesions that impair sensory function? These sensory areas of the hemisphere may be important in understanding swallowing functions and impairments. In fact, some studies report that many stroke patients with dysphagia have damage to the parietal lobe with associated sensory deficits.4 Primary sensory areas of the cortex have extensive interconnections with the motor areas of the cortex. Sensory function is deemed important in the control of voluntary movement. Beyond direct sensory loss, we should consider conditions in which the patient cannot interpret sensory information, for example, neglect. Patients with neglect may not respond to a stimulus in the swallowing tract (food or liquid bolus), not because of direct sensory loss, but because of a cortical deficit in processing and interpreting sensory information. In at least one study, hemispatial neglect was related to nonoral intake of food and liquid, but not severity of dysphagia, in patients evaluated 3 days after hospital admission for stroke.5 Unfortunately, these investigators did not interpret the association between neglect and nonoral intake. As a result, the presence of neglect may be related to feeding limitations rather than swallowing deficits leading to nonoral intake. More recently, increased systematic attention has been afforded sensory functions in swallowing and swallowing impairment.6–8 Continued emerging information and clinical observations suggest that impaired sensory functions may have a direct influence on swallowing functions. A better understanding of the role of sensory systems on swallowing function and impairment may lead to improved sensory-based interventions for dysphagia. The issues previously raised regarding hemispheric contribution to swallowing control also raise the question of whether such control is unilateral or bilateral. A traditional perspective is that patients with bilateral lesions often demonstrate the most severe and persistent dysphagia characteristics.9 Still, patients with unilateral hemisphere lesions may demonstrate dysphagia to varying degrees. Recent research using the technique of transcranial magnetic stimulation has suggested an interesting point of view on the hemispheric representation of swallowing function. Transcranial magnetic stimulation involves sending a magnetic current across the cranium over discrete hemisphere regions. These magnetic currents stimulate motor activity that is measured in various muscles by electromyography. This interesting work on the hemispheric control of swallowing function can be summarized as follows: 1. Swallowing motor functions are bilaterally represented in the hemispheres. 2. If the dominant hemisphere is impaired, a contralateral “backup” area may be available to facilitate recovery. 3. A form of cortical plasticity may occur over time, increasing the utility of the intact, nondominant hemisphere to control swallowing motor functions. 4. Bilateral strokes would result in the most tenacious dysphagias.10–13 Several issues must be addressed when considering dysphagia secondary to hemispheric strokes. These issues may be simplified into two general considerations: location of damage and functional consequences of the damage. These considerations are not mutually exclusive. Location of the damage may be important in understanding sensory and/or motor impairments and in understanding the severity and potential for recovery based on unilateral versus bilateral lesions. In clinical practice, information on lesion location often is not available at the time of the dysphagia evaluation. Therefore a strong reliance on the clinical examination of functional impairment after stroke may provide the best “road map” to understanding and perhaps predicting dysphagia characteristics. Table 5-1 provides a basic orientation to some of the functional impairments that may be clinically observed after impairment to various levels of the nervous system. At the hemisphere level, intent to swallow may be an important consideration. If the patient indicates such intent, a subsequent consideration would be motor initiation of the swallow. Patients with damage to premotor areas (e.g., supplemental motor cortex) may have generalized difficulty with motor initiation. The clinical picture may be that of a patient who holds a bolus in the mouth for an abnormally long period with associated movements that indicate the intent to swallow but without initiating a swallow. Finally, patients with hemispheric stroke may have significant communication deficits or cognitive deficits that reduce their ability to relate to the clinical examiner the nature of the dysphagia complaints. Patients who are asleep, lethargic, have waxing and waning alertness, or difficulty participating in the swallowing evaluation because of cognitive deficits present significant challenges to a valid evaluation of swallowing abilities. Also, the inability to describe swallowing difficulties may delay or hinder clinical evaluation and/or implementation of rehabilitation strategies. Figure 5-2 depicts general hemisphere areas that may be associated with various sensorimotor functions associated with swallowing. The left hemisphere is shown for descriptive purposes only. Box 5-1 presents various swallowing characteristics that may be associated with sensorimotor deficits after hemispheric stroke. A variety of swallowing deficits have been reported after hemispheric stroke. In general, hemispheric lesions (including both cortical and subcortical damage) contribute to many swallowing deficits (Box 5-2), including (1) poor initiation of saliva swallows (sometimes termed the dry swallow); (2) delay in initiation of the pharyngeal component of the swallow; (3) incoordination of the oral components of swallowing; (4) increased pharyngeal transit time and reduced pharyngeal constriction and clearing; (5) aspiration; (6) dysfunction of the pharyngoesophageal segment (cricopharyngeal muscle); and (7) poor relaxation of the lower esophageal sphincter. These collective observations indicate that hemispheric stroke can impair swallowing functions from the mouth to the stomach. Furthermore, a wide spectrum of swallowing deficits has been noted, ranging from impaired initiation of the swallow to poor transport of the bolus to aspiration into the airway. To date no report has emerged comparing specific sensorimotor stroke sequelae with specific swallowing impairments. However, the above list suggests that the array of potential swallowing deficits after stroke is extensive and may relate to the spectrum of poststroke sensorimotor impairments. Dysphagia is highly prevalent in acute stroke, with estimates that well over 50% of all patients are affected. However, the majority of acute stroke patients recover functional swallowing ability within the first 1 to 6 months after stroke, whereas swallowing problems develop in a small percentage of patients during the postacute period.14–16 These observations emphasize the importance of accurate identification and management of swallowing deficits in acute stroke patients. Furthermore, it is important to understand the factors that might predict persistent swallowing problems beyond the acute recovery period. The importance of this perspective is highlighted by the observation that acute and chronic swallowing problems in stroke patients are associated with many complications, including dehydration, malnutrition, aspiration, chest infections and, in some cases, death. Furthermore, dysphagia during acute stroke is associated with poor long-term outcome, including death and an increased rate of institutionalization.17 Perhaps the most obvious statement about dysphagia in stroke is that it changes over time. From that perspective, dysphagia intervention strategies should also change over time. Table 5-2 presents clinical considerations and decisions that may affect treatment planning over time. Early in the course of a stroke, focus should be given to basic decisions such as the safety of oral feeding versus the need for nonoral feeding routes, the presence of comorbid conditions such as pneumonia, malnutrition, or dehydration, and the overall medical condition of the patient. TABLE 5-2 Treatment Considerations and Decisions for Dysphagia After Stroke* The acute stroke patient is at greatest risk for dysphagia and morbidities associated with dysphagia. During the acute phase of stroke, patients are likely to demonstrate significant weakness contributing to reduced stamina and perhaps reduced mental status, including alertness and attention. These factors significantly limit any meaningful clinical (or other) evaluation of swallowing ability. Thus a conservative strategy would be to observe the patient’s status and postpone any in-depth assessment or intervention until the patient is more alert and has better endurance. Acute stroke patients also are at risk for respiratory abnormalities. Respiratory abnormalities range from basic weakness in expiratory muscles that might reduce cough effectiveness,18 to increased episodes of oxygen desaturation,19,20 to deviations in the respiratory rate,21 to alterations in the coordination between respiration and swallowing.22,23 Collectively, these respiratory deviations noted in acute stroke patients suggest an increased risk of aspiration of swallowed materials and pooled secretions and potential limitations in clearing aspirated secretions as a result of reduced cough efficiency. Given these potential risks, respiratory functions in the acute stroke patient should be evaluated as part of the comprehensive swallowing examination. Pneumonia is commonly noted in acute stroke patients. Pneumonia is a significant morbidity because it is related to both an increased number of hospital readmissions24 and short-term and long-term mortality.25 Causes of pneumonia in the poststroke patient are multifactorial; however, dysphagia, especially dysphagia accompanied by aspiration, is significantly related to the presence of pneumonia.16 In fact, dysphagia screening leading to early identification and treatment of swallowing deficits in acute stroke patients has been associated with a reduction in pneumonia rates.26 The presence of dysphagia after stroke may contribute to pneumonia in various ways. Although the focus is often on aspiration of orally ingested food and liquid, aspiration of pooled pharyngeal secretions also may contribute to chest infection. Aspiration of secretions may be especially problematic in the acute stroke population because oral bacteria colonization is prominent in these patients.27 Patients dependent on tube feeding, specifically nasogastric tube feeding, may have a higher degree of bacterial colonization than patients who feed orally.28 In fact, at least one study has reported that stroke patients who were completely dependent on nonoral feeding (e.g., nothing by mouth) demonstrated higher rates of respiratory infections than did stroke survivors who were feeding orally.29 One implication of these findings is that reduced frequency of swallowing contributes to an increased risk of aspirating pharyngeal secretions in the presence of higher rates of bacterial colonization within the swallowing mechanism. This premise is supported by treatment studies demonstrating that swallowing therapy30 and strategies to improve oral hygiene31 reduce the incidence of pneumonia in stroke patients. Thus the dysphagia clinician should consider more than aspiration of food and liquid when providing swallowing interventions to patients after acute stroke. Nutritional deficits are prevalent among stroke patients on admission and may worsen during hospitalization. On admission, the prevalence of such deficits has been estimated at approximately 16%; this figure increases to 22% to 26% through discharge from acute care.32–34 Nutritional decline continues beyond acute care. The prevalence of nutritional deficits in stroke patients at admission to rehabilitation approximates 50%.35 At approximately 1 month after stroke, nutritional status begins to improve and continues to improve up to 4 months after stroke. In the acute stroke patient nutritional deficits are not overtly linked to dysphagia.33,36 However, later during the rehabilitation period and thereafter, swallowing and/or feeding difficulties may contribute to the maintenance or increase in poor nutritional status.37 Still, some suggest that poor nutrition during the acute phase of stroke contributes to poor longer term functional outcomes.38 Nutritional evaluation and intervention are outside the scope of practice for most dysphagia clinicians. However, all dysphagia clinicians should be aware of the potential impact of swallowing and feeding abilities on nutritional status and participate in multidisciplinary health care teams, including nutritional specialists. As the patient’s condition improves and more active rehabilitation is initiated (usually well within the first month after stroke), dysphagia treatment strategies also may change. One consideration is spontaneous resolution of dysphagia as the patient recovers from the effects of acute stroke. Although many stroke patients have some degree of recovery in swallowing ability, estimates of persisting dysphagia range from 11% to 50% at 6 months after stroke.14,39 During this period of improvement the patient with persistent dysphagia is likely to be engaged in active swallowing rehabilitation. By this time a decision about oral or nonoral feeding has already been established and comorbid conditions are often under medical control. Of importance to active dysphagia rehabilitation are various patient issues and the nature of the swallowing deficit. If the patient is able to participate in active rehabilitation and is motivated, direct and intense swallowing therapy is expected to produce significant benefit. Benefits from swallowing therapy extend beyond improved swallowing abilities to include reduced pneumonia rates and improved nutritional status.29,40–42 Decisions about therapy technique(s) selection depend in large part on the specific dysphagia characteristics demonstrated by individual patients (see Chapter 14). Chronic dysphagia is reported in some stroke survivors, although no study has documented the prevalence of dysphagia in stroke patients beyond 6 months after stroke. Typically, if the swallowing deficit persists beyond 6 months, it is considered chronic. Available reports indicate that stroke patients with chronic dysphagia can benefit from intense therapy.43–45 Such therapies are typically active and directed at changing specific physiologic features of the swallowing deficit. Another form of cortical impairment that can affect swallowing ability is the category of progressive diseases known as dementia. Several types of dementias have been described; the most frequent is Alzheimer’s disease. Other forms of dementia include dementia caused by cerebrovascular disease, Lewy body dementia, frontotemporal dementia, alcoholic dementia, and metabolic and/or nutritional dementia.46 The hallmark of all dementias is a progressive deterioration in cognitive abilities, including memory, judgment, abstract reasoning, and personality changes. Other cortical disturbances such as apraxia and/or aphasia might be noted. Swallowing deficits are well documented in advanced dementia.47–50 Persistent weight loss may be the first indication that patients with dementia have a significant swallowing problem; however, weight loss may not be directly related to feeding or swallowing difficulties.49,51 As a result, such individuals are at significant risk for nutritional deficits that may further compromise their health status. Pneumonia is a common cause of death in patients with dementia.50 Although dysphagia, including aspiration, is associated with pneumonia in this population,51 it is not the only contributing factor and may not be the critical contributing factor.49,50,52 General characteristics of swallowing deficits in dementia are listed in Box 5-3. Prominent on this list is the presence of oral-stage dysfunction. It has been suggested that certain oral aspects of swallowing are under volitional motor control. The generalized cognitive impairments in dementia may contribute to deficits in volitional motor control and hence oral aspects of dysphagia. These may be characterized by lack of initiation of the swallow in which the patient holds food in the mouth, has incoordinated oral control of food and liquid, and/or delayed initiation of the oral component of the swallow, thus prolonging mealtimes. Although the majority of dysphagia information in dementia is derived from studies of patients in advanced stages of the disease, patients with mild-stage dementia also demonstrate feeding and swallowing deficits.53 Box 5-4 summarizes the salient findings regarding feeding and swallowing abilities in mild-stage dementia. These impairments are similar to, though not as severe as, those reported in more advanced stages of the disease. Specifically, patients with dementia demonstrate an overall slowing of the swallowing process from the oral aspects of food manipulation through the response of the pharynx accepting the bolus. This slowing of the swallowing process can have direct consequences for longer mealtimes and hence increase the risk of declining nutritional status. In addition, slowing of the pharyngeal response in swallowing may reduce airway protection, resulting in an increase of coughing and choking behaviors during mealtimes.
Adult Neurologic Disorders
PRELIMINARY CONSIDERATIONS: SWALLOWING SYMPTOMS AND NEUROLOGIC DEFICITS
Brief Overview of Functional Neuroanatomy Relative to Swallowing Functions
Level
Motor
Sensory
Cortical
Subcortical (basal ganglia)
Brainstem
Cerebellum
Refinement
Peripheral nerves
Sensory conduit
Muscles and sensory receptors
Effect movement
Sensation reception
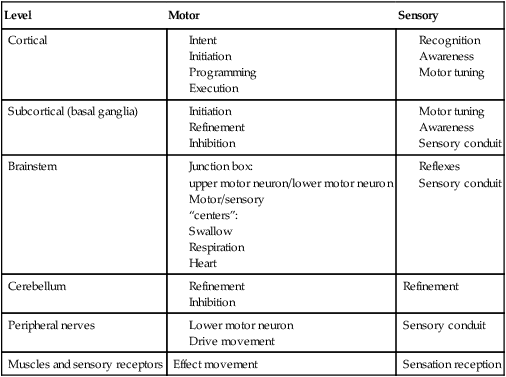
CORTICAL FUNCTIONS
Cortical Functions and Swallowing Impairment
Issues of Unilateral versus Bilateral Hemispheric Lesions
SWALLOWING DEFICITS IN HEMISPHERIC STROKE SYNDROMES
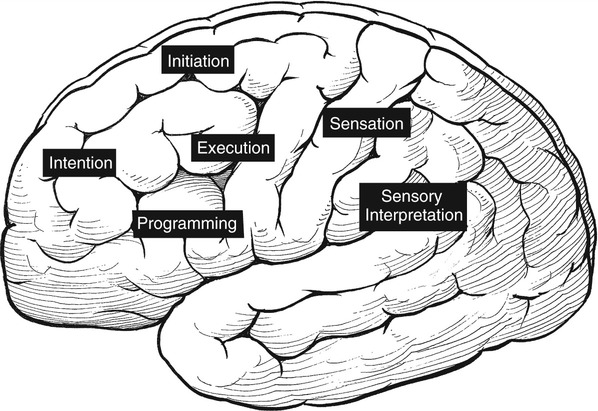
Treatment Considerations
Considerations
Decisions
Acute (0-1 Month)
Most comorbid conditions
How to avoid or minimize complications
Resolving dysphagia
Need and readiness for therapy
Malnutrition
How to maintain and improve nutrition
Improving (1-6 Months)
Patient more stable with better endurance
Need and readiness for therapy
Comorbid conditions often under medical control
Type of therapy
Feeding routes established for most
Malnutrition may still be a factor
Chronic (After 6 Months)
Feeding routes more established
Therapy or no therapy (prognosis?)
Patients eating orally may have impaired swallow
Type of therapy
Compensations that interfere with swallow
Impact of prior therapy
SWALLOWING DEFICITS IN DEMENTIA
Adult Neurologic Disorders