Chapter 62A Resectional techniques
Pancreaticoduodenectomy, distal pancreatectomy, segmental pancreatectomy, total pancreatectomy, and transduodenal resection of the papilla of Vater
Overview
The modern surgeon can certainly appreciate the trepidations felt by early surgical pioneers faced with such a clinical situation. Early series on pancreatic operations for cancer published in the late 1960s reported postoperative morbidity rates of 60% and mortality rates approaching 25%, with dismal long-term outcomes. Consequently, Crile (1970) opined that patients would be better served by a bypass procedure rather than putting them through a futile and risky resection. Such a nihilistic view was the prevailing attitude prior to the 1980s, to the point that surgeons asked themselves whether pancreatoduodenectomy (PD) should be abandoned as treatment for pancreatic cancer (van Heerden et al, 1981).
In the ensuing decades, however, a dramatic decline was seen in surgical mortality and morbidity rates. High-volume pancreatic surgical centers are consistently reporting mortality rates of less than 2% and morbidity rates of 36% (Büchler et al, 2003; Cameron et al, 2006; Wagner et al, 2004). Certainly, continual improvements in surgical technique have played a role, but credit cannot be claimed solely by the surgical profession; significant advances were achieved in tandem in other fields that included better understanding of pancreatic diseases, advances in diagnostics, better patient selection, and improvements in perioperative care. Perhaps one of the main contributors to this phenomenon was the establishment of high-volume centers (Birkmeyer et al, 2003; Fong et al, 2005). Such centers tend to boast larger facilities and therefore have a broader range of specialist- and technology-based services, with better staffed intensive care units. This also implies that complications are better recognized and managed.
Although the question of the safety of pancreatic resection has been effectively addressed, the long-term outcome is more controversial as far as pancreatic adenocarcinoma is concerned. The concept of cure following “curative” resection has recently been challenged (Gudjonsson, 1995), but surgical resection is the only therapy that offers significantly increased survival. A randomized multicenter trial affirmed the superior results of resection on survival compared with various forms of nonsurgical therapy (Imamura et al, 2004). The median survival following resection was 14.3 months, whereas patients who did not undergo surgery died at 4.9 months (Conlon et al, 1996); 10% to 30% of patients will be true 5-year survivors (Cameron et al, 2006; Wagner et al, 2004). Furthermore, although pancreatic cancer is the most common of the periampullary tumors, cancers of the ampulla, duodenum, and distal bile duct, as well as intraductal papillary mucinous neoplasm–associated adenocarcinoma, have a better long-term survival following curative PD (Poultsides et al, 2010). In addition, more and more cystic pancreatic and endocrine tumors are diagnosed today, and simple enucleation or segmental resection of the tumors at the appropriate stage can cure the disease. Even with advances in multimodal treatment, surgery remains the centerpiece of the treatment algorithm for pancreatic cancer because no truly effective chemotherapeutic agents for treating nonresectable disease have been developed (Gillen et al, 2010; Postier, 2003).
Technical refinements have led to a variety of surgical techniques that have allowed a more individualized, disease-directed approach. These modifications were partly responsible for the decline in surgical morbidity. This chapter focuses on some of these surgical techniques, as practiced in our institution, as well as some of the more pertinent perioperative issues for patients subjected to pancreatic resection. Surgical techniques specific for the treatment of acute pancreatitis (open and minimally invasive necrosectomy) and chronic pancreatitis (duodenum-preserving pancreatic head resection) are described in detail in Chapters 54 and 55B.
Preoperative Workup and Management
Diagnosis and Staging of Disease
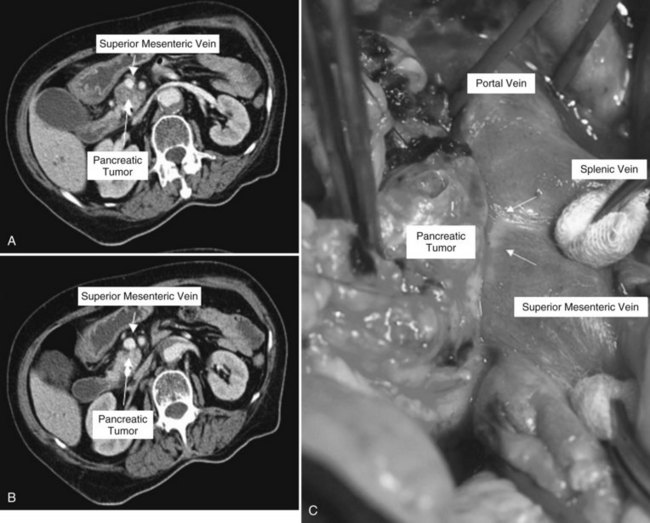
Clinical staging should reliably define the extent of disease, thereby avoiding unnecessary intervention and the accompanying morbidity, mortality, and diminished quality of life in patients with advanced disease (Spanknebel & Conlon, 2001). Although the tumor-node-metastasis (TNM) staging system is most often used in clinical trials, physicians in practice typically classify patients as having resectable, locally unresectable, or metastatic disease (Haller, 2002). Resectable pancreatic cancer is universally defined as a pancreatic tumor without evidence of involvement of the superior mesenteric artery (SMA) or the celiac axis and no evidence of distant metastasis. Tumors with infiltration of one of these central arteries are defined as locally irresectable, but those with infiltration of the superior mesenteric vein or abutment of visceral arteries are defined as borderline resectable (Adler et al, 2007; Katz et al, 2008). Portal vein (PV) involvement, although somewhat controversial, should not be a contraindication for surgery; today, this is still center dependent. In general, PV involvement does not preclude an R0 resection (Fig. 62A.1).
Imaging
Helical computed tomography (CT) scanning has been established as the single most effective initial staging study (Freeny, 2001; Klauss et al, 2008) and is often used as the entry point to a management algorithm. The findings then dictate the subsequent management, including additional diagnostics (see Chapter 16).
The previous selling point of magnetic resonance imaging (MRI) (see Chapter 17) was its being a one-stop modality that combines pancreatography, cholangiography, and angiography and also assists in the evaluation of tumors. However, MRI is being progressively overshadowed with the advent of the multidetector CT, which has nearly the same offerings. As such, MRI offers no added benefit over CT for evaluating pancreatic cancer, except perhaps in patients in whom a contrast-enhanced CT cannot be performed (White et al, 2003), and MRI seems to be superior for the differential diagnosis of pancreatic cystic lesions (see Chapter 57).
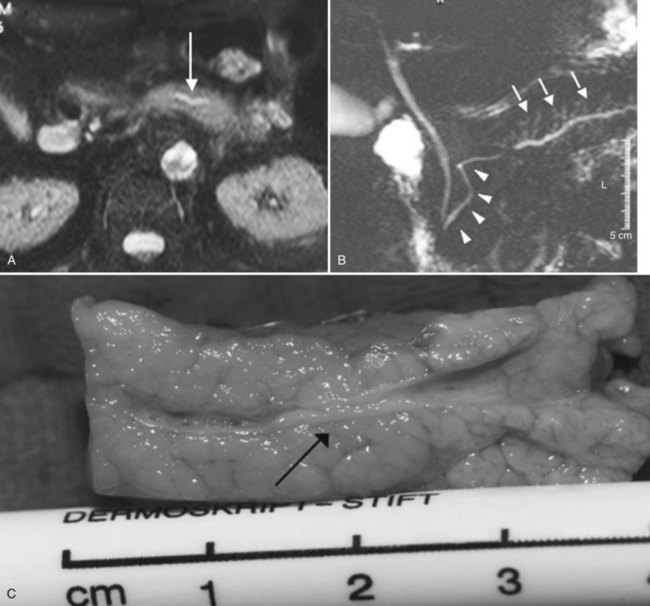
MR cholangiopancreatography (MRCP) offers a noninvasive delineation of the pancreatic and biliary ducts. It detects pancreatic or ampullary carcinoma by demonstrating the effect of a space-occupying lesion on the ducts, namely obstruction or displacement (Fig. 62A.2). The classic feature is the “double-duct” sign. However, even a strictly defined double-duct sign is only 80% to 85% specific for malignancy (Menges et al, 2000). More recent applications include secretin-enhanced MRCP, which can improve pancreatic duct and side-branch delineation. Furthermore, such pharmacologic stimulation of pancreatic juice secretion can potentially allow the evaluation of pancreatic flow dynamics and assessment of pancreatic exocrine function (Kalra et al, 2003).
Endoscopic ultrasonograpy (EUS) allows a sonographic transducer to be placed in close proximity to the pancreas (see Chapter 14). In doing so, interference from overlying bowel gas is eliminated, thus allowing higher frequencies to be used and resulting in markedly improved resolution of the imagery. Consequently, EUS is more sensitive in detecting smaller lesions (<20 mm), with a sensitivity in the range of 93% to 100% (Tamm et al, 2003). In a meta-analysis of studies comparing staging by EUS to results with other modalities, EUS without fine needle aspiration (FNA) more accurately predicted T stage, N stage, and PV involvement than did CT scanning. EUS also allows image-guided FNA of the primary tumor and the regional lymph nodes without the possible risk of tumor seeding along the needle tract, unlike FNA via the percutaneous route (Gress et al, 2001).
EUS-guided FNA biopsy has been reported to have a high sensitivity (93%) and specificity (100%) (Wiersema, 2002); however, EUS with FNA is only of diagnostic value if histology confirms a pancreatic tumor. The major limitations of this technology are operator dependence and a limited field of visualization for the detection of distant metastasis. In fact, in resectable pancreatic cancer, but also in cystic or endocrine tumors, preoperative biopsies are of no value (Hartwig et al, 2009a). Therefore, we use this modality on a highly selective basis, such as in obtaining tissue biopsy for patients scheduled for neoadjuvant therapy with locally advanced pancreatic cancer, or before palliative chemotherapy is applied in metastatic disease.
With the advent of MRCP, EUS, and multidedector CT with multiplanar three-dimensional (3D) reconstruction, the role of endoscopic retrograde cholangiopancreatography (ERCP) as a diagnostic tool has become increasingly limited. Furthermore, it has potentially high complication rates compared with other procedures (Ciocirlan & Ponchon, 2004). Such concerns have been summarized in a recent report by the American Society of Gastrointestinal Endoscopy (ASGE, 2003). In light of these data, the current view is that purely diagnostic uses of ERCP should be limited, a view affirmed by the National Institutes of Health (NIH) State of the Science Conference Statement (Cohen et al, 2002), which recommended that for patients with pancreatic or biliary cancer, the principal advantage of ERCP is palliation of biliary obstruction when surgery is not elected.
General Preoperative Patient Assessment
Pancreatic resections exert a significant physiologic stress on patients, many of whom are elderly; the peak incidence of pancreatic cancer falls in the 65- to 75-year age group (Lankisch et al, 2002). With the improvement of the safety profile of pancreatic operations, many centers have offered surgery to octogenarians, although in such patients the incidence of comorbidities is higher.
In our center, we found that systemic complications, chiefly cardiopulmonary complications, have become the main cause of operative death (Büchler et al, 2003). As such, we routinely subject our patients to a rigorous preoperative assessment of their cardiac, pulmonary, and renal functions. Cardiopulmonary exercise testing has been shown to more accurately evaluate the ability of the cardiorespiratory system to deliver oxygen under stress (Davies & Wilson, 2004). We have also included a pulmonary function test in our algorithm. Optimization of afterload and myocardial contractility take equal importance, and pulmonary artery catheters are occasionally inserted to facilitate this. The operation will only proceed when we can achieve an optimal hemodynamic profile.
Perioperative Management (See Chapters 22, 23, and 24)
Perioperative Anticoagulation
Meta-analysis of the role of low-molecular-weight heparin (LMWH) in the prevention of venous thromboembolic events in general surgery has shown that LWMH can significantly reduce the incidence of asymptomatic deep vein thrombosis (DVT), clinical venous thromboembolism, and pulmonary embolism, with a trend toward a reduction in overall mortality rate (Mismetti et al, 2001). Consequently, we routinely administer LMWH in a prophylactic dose to our patients, starting the evening before the day of surgery, until the patient is ambulant postoperatively. In addition, our patients are prescribed compression stockings to wear intraoperatively and for their entire inpatient stay because such stockings reduce pooling of blood in deep veins by mechanically preventing venous distension (Morris & Woodcock, 2004) and are thus a simple and cost-effective method of DVT prophylaxis.
Antibacterial Prophylaxis
Antibacterial prophylaxis is instrumental in the reduction of infection-related morbidity with clean-contaminated procedures (de Lalla, 2002); as such, it is recommended for all patients who undergo hepatobiliary or pancreatic surgery (Sganga, 2002). Drugs with antianaerobic activity are added if anaerobe encounter is anticipated during the procedure, in particular procedures involving the gastrointestinal (GI) tract. The general guideline is to use the highest licensed dosage of the chosen antimicrobial agent, administered at induction of anesthesia so as to achieve high peak tissue concentration at the site of the wound before the first incision, which should be maintained until the time of closure (Polk et al, 2000). For our pancreatic operations, in addition to 500 mg metronidazole, we routinely give 4 g mezlocillin, an ureidopenicillin with activity against both gram-positive and gram-negative organisms, including enteric bacilli. We generally do not give postsurgical doses unless the patient has had a recent bout of cholangitis or if the bile was found to be turbid or purulent intraoperatively. In any event, in all procedures that require entry into the biliary tract, bile is sent for microbiologic examination to guide postoperative antimicrobial treatment if the need arises.
Octreotide Analogues
The pancreatoenteric anastomosis is considered the Achilles heel of PD, a testimonial to the potentially disastrous sequelae of life-threatening intraabdominal sepsis and hemorrhage (Stojadinovic et al, 2003) in the event of a pancreatic leak. The secretion capacity of the remnant gland has been found to be a determining factor for the development of a leak because continuous pancreatic secretion has been hypothesized to hinder healing of the pancreatic stump. This led to the hypothesis that by reducing exocrine secretion, perhaps the incidence of pancreatic fistula could be reduced accordingly.
Octreotide, the octapeptide analogue of somatostatin, is a powerful inhibitor of pancreatic exocrine secretion. A number of randomized prospective trials have examined the role of prophylactic, perioperative octreotide and its impact on outcome after pancreatic surgery. The results seemed to display an intercontinental difference: level 1 studies from Europe generally showed that perioperative somatostatin has a protective effect, whereas all the North American studies failed to demonstrate any benefit. A recent systematic review of this topic came to the conclusion that the prophylactic administration of somatostatin or its analogue does not uniformly reduce the incidence of pancreatic anastomotic leak, overall morbidity, or mortality after pancreatic resection (Li-Ling & Irving, 2001). Based on the findings of the trials conducted by our center (Büchler et al, 1992; Friess et al, 1995), we believe that some benefits may still be reaped from the use of prophylactic somatostatin in high-risk patients (soft pancreas, small duct), in whom a PD is performed. If the pancreas is deemed to be high risk by the surgeon, because of a soft consistency and a pancreatic duct size of less than 2 mm in diameter, somatostatin is applied intraoperatively (200 µg subcutaneously), followed by three daily doses of 200 µg octreotide for 5 days. Other indications for applying somatostatin are when segmental resections or enucleations must be performed in patients with a soft pancreas, as is frequently observed with cystic or endocrine lesions.
Preoperative Biliary Drainage
Patients with pancreatic cancer who have jaundice are also at risk for associated coagulopathy, malabsorption, malnutrition, and immune dysfunction. Using a multiple-variant regression analysis, we had previously determined that jaundice (bilirubin level >5.8 mg/dL) is a significant risk factor for postoperative hemorrhage (Martignoni et al, 2001). This calls into question the role of preoperative biliary drainage (PBD) in reducing surgical morbidity through the normalization of liver function and the correction of coagulopathy. Sewnath and colleagues (2002) found in a meta-analysis no difference in the overall death rate among patients who had PBD and those who had surgery without PBD. Instead, the overall complication rate was adversely and significantly affected by PBD, and the hospital stay was also prolonged, so they concluded that PBD carries no benefit. Recent studies also demonstrate that routine PBD should not be performed because of the high rate of procedure-associated complications (Mezhir et al, 2009; van der Gaag et al, 2010). We concur with the opinion that ERCP should only be undertaken with therapeutic intent and believe that PBD as a routine practice should be halted. We would refer a patient for PBD in the presence of cholangitis or other severe complication of jaundice that would preclude a safe, early resection. An absolute indication for PBD is jaundice that requires induction therapy prior to surgical extirpation.
Fast-Track Surgery
Studies on so-called fast-track GI surgery have shown that epidural analgesia, combined with an intensive and standardized regimen of early feeding and mobilization, can reduce hospital stay (Basse et al, 2002). Epidural analgesia has been found to have many attributes, including a shorter duration of postoperative ileus, attenuation of the stress response, fewer pulmonary complications, improved postoperative pain and mobility, and patient perception of a quicker recovery (Fotiadis et al, 2004). Thoracic epidural analgesia is of particular benefit to patients with a high risk of cardiac or pulmonary morbidity, and it can reduce hospital stay and costs in this subgroup of patients.
Resectional Techniques
Pancreatoduodenectomy
Ductal adenocarcinoma is by far the most prevalent tumor of the pancreas, with a predominant localization within the pancreatic head (78%) (see Chapter 58A, Chapter 58B, Chapter 59 ; Schäfer et al, 2002). Furthermore, adenocarcinoma of the body or tail is seldom resectable on presentation. It is thus not surprising that PD is the best-studied pancreatic surgical procedure.
Technique
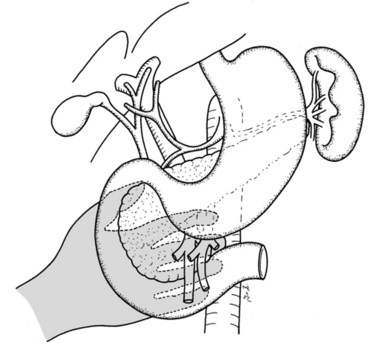
In thin patients, we prefer a midline incision beginning from the xiphoid process, but we perform a rooftop incision in patients with a short torso and a wide abdominal girth. After we have ruled out liver metastasis, the peritoneal surfaces are carefully inspected for metastatic deposits. Particular attention is paid to the pelvis for drop metastasis and to the root of the mesentery for evidence of tumor extension or matted nodes. A wide Kocher maneuver is then performed to assess the retroperitoneum and appraise the tumor and its relationship to the SMA (Fig. 62A.3). The mesenteric artery is inspected from the ligament of Treitz and dissected on its right hemicircumference to exclude any tumor infiltration of the artery. This technical approach, the so-called artery-first approach (Fig. 62A.4; Weitz et al, 2010), helps avoid R2 resections because any infiltration of the central arteries can be diagnosed early during exploration. We proceed with the resection only if we find no evidence of infiltration of the celiac trunk and SMA.
Cholecystectomy is performed in a typical fashion. The cystic duct is traced to its origin from the common bile duct (CBD), which is transected just cephalic to this point. Extreme care must be taken to avoid any iatrogenic injury to the right hepatic artery, which usually runs posterior to the hepatic duct (see Chapter 1B; Skandalakis et al, 2004). In addition, it is important to assess for an aberrant right hepatic artery, present in 20% of patients, by palpation of the posterior aspect of the hepatoduodenal ligament. The cystic artery is isolated and closed with ligation.
If preservation of the pylorus is planned, which is our usual practice, the right gastric and right gastroepiploic arteries are divided and the duodenum is skeletonized 2 cm beyond the pylorus. The suprapancreatic portion of the PV will now be widely exposed, and two stay sutures are similarly placed on the superior border of the pancreas; these sutures at the superior and inferior pancreatic borders also serve to ligate the superior and inferior pancreatic vessels respectively, running longitudinally in the parenchyma. Hence bleeding from the cut edges is reduced after transection. A tunnel is cautiously created between the SMV-PV trunk posteriorly and the pancreatic neck anteriorly. A silicon drain is then insinuated into this tunnel to loop up the neck (Fig. 62A.5).
Because the medial margin is the most crucial with regard to tumor infiltration and the R status of the resection (Esposito et al, 2008), we now perform the uncinate process–first approach (Hackert et al, 2010). After having exposed and evaluated the SMA, the ligament of Treitz is mobilized, and the mesenteric branches to the fourth part of the duodenum are divided to allow it to be delivered into the inframesocolic compartment under the SMA. The mesenteric window is extended to the proximal jejunum, which is transected at a point that allows a tension-free loop of jejunum to be brought into the supramesocolic compartment in a retrocolic fashion through a window created on the right side of the middle colic pedicle. The dissection is performed along the SMA and SMV with bipolar cautery and clips.
Standard Pancreatoduodenectomy Versus Pylorus-Preserving Pancreatoduodenectomy
In addition to the known postoperative complications associated with PD, the performance of a distal gastrectomy in the standard procedure also brings with it the possible long-term morbidities of gastric dumping, marginal ulceration, and bile-reflux gastritis (Poon & Fan, 2001). First introduced by Kenneth Watson (1944), it was not popular until 1978, when Traverso and Longmire (1978) reintroduced it. They hypothesized that the preservation of the stomach and pyloric function would improve GI function and hence reduce the morbidities associated with a gastroenterostomy. Indeed, better long-term digestive function and quality of life have been found after pylorus-preserving pancreatoduodenectomy (PPPD) in some retrospective studies. To retain a functioning pylorus, the entire stomach, 2 cm of the first part of the duodenum, and the neurovascular supply to both are preserved. Following the division of the right gastric and right gastroepiploic arteries at their origin, the duodenum is skeletonized 2 cm distal to the pylorus (Farnell et al, 2001), taking care to preserve the nerve of Latarjet. The duodenal bulb is then transected.
A recent meta-analysis demonstrated that the classical Kausch-Whipple procedure and the pylorus-preserving Whipple procedure are comparable with regard to perioperative morbidity and mortality and long-term outcome (Diener et al, 2008b). Hence, in the light of the available evidence, PPPD should be designated the “standard” procedure for pancreatic and periampullary neoplasm because no study to date has demonstrated any advantage to the removal of the stomach and the proximal duodenum (Bell, 2004). Hemigastrectomy should be performed only when gastric or proximal duodenal invasion or grossly abnormal peripyloric nodes are evident.
Vascular Resection
Over 30 years ago, Fortner (1973) reasoned that a more radical resection should improve survival by improving tumor clearance, specifically that tumor adherence to the PV or SMV, often regarded as a criterion of unresectability, could be overcome by en bloc resection of the involved vessels. Randomized evaluation of portal and superior mesenteric vein resection in patients with tumors adherent to these vessels is difficult because considerable intersurgeon variation in the interpretation of adherence is likely. Thus no randomized trial evaluates this topic.
A recently published systematic review (Siriwardana & Siriwardena, 2006) evaluated 52 manuscripts with 6333 patients in whom pancreatic resection was performed for pancreatic cancer; 1646 of these patients (26%) underwent synchronous portal and superior mesenteric vein resection. The median number of resections per publication evaluated was 82; of those, 23 patients underwent portal and superior mesenteric vein resection. The proportion of portal and superior mesenteric vein resection per publication varies widely, ranging from 2% to 77%, which mirrors the various treatment approaches of pancreatic cancer in different institutions today.
The assessment of pooled data is critically influenced by the quality of the single reports. The reports included have been published over the last 25 years, and the standards of perioperative care, surgical technique, and adjuvant therapy have developed dramatically. Thus the wide variations of outcome parameters—perioperative death, operative time, blood loss—mirror the nonhomogenous collective included in this review. However, the long-term survival rate demonstrates that resection of the portal or superior mesenteric vein is a potentially curative operation. It seems that patients who need a portal or superior mesenteric vein resection for cure include a high percentage of patients with a positive nodal stage. Because adjuvant treatment has been proven to increase survival but has only recently been introduced as standard care for patients with pancreatic cancer, the survival rates of patients with portal or superior mesenteric vein resection today should be even better than those reported in this review (Neoptolemos et al, 2004; Oettle et al, 2007).
In addition, surgical expertise has improved over the past few decades, and the quality of high-volume centers—especially for technically demanding surgeries, such as pancreatic resection—has been proven (Birkmeyer et al, 2003; Büchler et al, 2003). Thus it is not surprising that perioperative morbidity and mortality rates reported for pancreatic resections with portal or superior mesenteric vein resection are identical to those without it (Bachellier et al, 2001; Carrere et al, 2006; Leach et al, 1998; Nakao et al, 2006; Riediger et al, 2006; Wagner et al, 2004).
Tumor infiltration of the SMA, celiac trunk, or hepatic arteries are well-accepted criteria for unresectability of pancreatic adenocarcinoma. However, because adherence of tumors to the arteries does not automatically mean infiltration—in fact, the arterial wall is resected much longer than the venous wall—several published series of pancreatic resections included resection and reconstruction of visceral arteries (Hartwig et al, 2009a; Li et al, 2004; Sasson et al, 2002; Varadhachary et al, 2006). A recent review states that in 15% of pancreatic operations in which portal or mesenteric vein resection had to be performed, arteries were also resected. These included the common hepatic artery (50%), SMA (20%), celiac axis (10%), and other arteries (Siriwardena & Siriwardena, 2006).
Multivisceral Resections
The benefit of radical surgical resection of contiguously involved structures for locally advanced pancreatic cancer has been addressed in several reports (Hartwig et al, 2009; Sasson et al, 2002). In fact, about 35% of patients are seen initially with locally advanced pancreatic cancers with involvement of surrounding structures and organs. Although several reports in the past showed that morbidity of extended resection was increased and survival benefit limited, more recent publications demonstrate that en bloc resection of contiguously involved organs can be performed safely (Sasson et al, 2002). No difference was reported with regard to perioperative morbidity (35%) and mortality (3%) compared with standard resection. Although operation time is clearly longer because of the extended resection—which includes mesocolon, colon, adrenal glands, liver, and stomach—blood loss and hospital stay are not different from what is observed after the standard procedure. The main aim of the extended procedure must be an R0 resection because this is the most important predictor of long-term survival (Wagner et al, 2004). The survival rate after resection of mesocolon and colon, as well as stomach, is not significantly decreased compared with the standard procedure (Sasson et al, 2002). The 5-year survival rate was 16%, and the median survival of 26 months is much better than the 6 to 9 months reported for patients who are not resected at all.
Similarly, another study demonstrated that patients with pancreatic adenocarcinoma of the body or tail need extended resection for contiguous organ involvement more frequently because these tumors are generally detected later and have therefore reached a greater size than cancers of the pancreatic head by the time of diagnosis. The median survival in these patients was 15.9 months, and the survival at 5 and 10 years was 22% and 18%, respectively. In our series of more than 100 patients with multivisceral resections, we showed that perioperative mortality rate and long-term outcome are similar to those of standard resections (median survival, 20 months), but that the morbidity is increased. Thus these patients need careful postoperative observation in an experienced pancreatic center (Hartwig et al, 2009).
Lymphadenectomy
The rationale for extended lymphadenectomy is that lymph node studies have confirmed positive lymph nodes may be found outside the confines of the standard dissection (Cubilla et al, 1978). Even for small cancers, lymph nodes of the paraaortic region, between the celiac trunk and the origin of the inferior mesenteric artery, frequently harbor metastases; it has been suggested that these should be dissected en bloc during radical resections (Nagakawa et al, 1993). These revelations initiated a movement of extended lymphadenectomy among Japanese surgeons. A few studies reported similar surgical morbidity and mortality rates, but improved survival results were seen with extended surgery compared with the standard PDs (Ishikawa et al, 1993). However, these were all retrospective, nonrandomized studies.
What constitutes extended lymphadenectomy is still widely debated; this is epitomized by the multitude of terminologies used in the literature. In an attempt to harmonize this subject, a consensus conference on the surgical treatment of pancreatic cancer took place in Castelfranco Veneto, Italy (Pedrazzoli et al, 1999). This consensus provided a standardized definition of the different extent of lymphadenectomy that used the Japanese Pancreas Society rules for the study of pancreatic cancer to define the lymph node stations to be removed for the various procedures (Japan Pancreas Society, 1993). The three stages of radicality for PD have been named standard, radical, and extended radical, depending on the nodal stations removed. For cancers of the body or tail, according to the extent of lymphadenectomy, two different procedures are identified: standard and radical. It is hoped that this standardization of surgical procedures will facilitate comparison of results between different centers.
Between 1988 and 1999, eight retrospective studies were published on the extent of lymphadenectomy for PD. The data on survival are largely heterogeneous, but neither morbidity nor mortality seems to be altered. Altogether, two studies reported on morbidity rates, whereas five studies reported perioperative mortality rates. Especially in Japan, extended lymphadenectomy was widely used in the late 1980s and 1990s because of the initial reports of Ishikawa and colleagues (1988) and Manabe and colleagues (1989), who advocated an extension of the PD. In these studies, survival rates were significantly increased in the extended lymphadenectomy groups. Interestingly, some long-term survivors were seen in the extended lymphadenectomy groups, but none (Manabe et al, 1989) or very few (Ishikawa et al, 1988) of the patients in the standard lymphadenectomy arm survived for more than 5 years.
In terms of operative techniques, total pancreatectomies were used in many patients, which may have also altered the outcome. Interestingly, the largest retrospective studies (Hirata et al, 1997; Mukaiya et al, 1998
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree