Chapter 44 Recurrent pyogenic cholangitis
Overview
Recurrent pyogenic cholangitis (RPC) is characterized by repeated bacterial infections of the biliary tract as a result of stones and bile duct strictures (see also Chapters 7, 11, 39, and 43). This condition was first described in Hong Kong by Digby in 1930, and the name recurrent pyogenic cholangitis was first coined by Cook in 1954. RPC has many synonyms, including Oriental cholangiohepatitis (Stock & Fung, 1962), Hong Kong disease (Mage & Morel, 1965), intrahepatic stones (Wen & Lee, 1972), hepatolithiasis (Nakayama et al, 1980), primary cholangitis (Choi et al, 1981), and Oriental infestational cholangitis (Seel & Park, 1983). It is prevalent in East Asia (Balasegaram, 1972; Chang & Passaro, 1983; De & Acharya, 2001; Maki et al, 1964; Nakayama et al, 1980; Ong, 1962; Seel & Park, 1983). With ease of travel and increasing migration from Asian countries, RPC is being encountered more frequently in Western countries, particularly in cities where Asian emigrants congregate (Al-Sukhni et al, 2008; Harris et al, 1998; Nguyen et al, 2009). RPC should not be regarded as a curiosity peculiar to Asia but rather as a disease that may affect Asians wherever they live.
RPC affects the young and the elderly of those who were of the lower socioeconomic classes growing up. There is no gender difference in incidence. In the past, RPC was one of the most common surgical emergencies, but the incidence has declined in recent years, particularly in urban centers (Nakayama, 1982). The overall incidence of RPC has decreased in Japan, from 50% of the cases of biliary stones in the pre–World War II years to 20% (Nakayama et al, 1980) despite advances in diagnostic techniques. In Hong Kong today, 12% of biliary stone cases are due to RPC (Fan et al, 1991a).
Etiology And Pathogenesis
The exact etiology of RPC continues to be elusive and is probably multifactorial. The likely initiating event is the establishment of infection by bowel microorganisms in the small biliary radicles (see Chapter 43). Experimental and clinical studies (Nakayama et al, 1980; Ong, 1962) indicate that the organisms isolated from portal vein blood, common duct bile, and liver biopsy specimens are predominantly of bowel origin (see Chapter 11). Although bowel organisms may reach the liver under ordinary circumstances, clinical infection does not occur except when bowel infection is severe, the organism is of particular virulence, or the host defense in the liver is compromised. In RPC, numerous organisms may enter the portal vein during a serious attack of enteric infection, which was previously common in Asia. As this condition often affects the lower socioeconomic classes, malnutrition and perhaps infection by flukes and worms may reduce the capacity of the liver to clear enteric bacteria effectively.
Whether stones or strictures develop first is not clear (see Chapter 39). Endoscopic retrograde cholangiopancreatography (ERCP) of the bile ducts in RPC (Lam et al, 1978) suggests that structural changes may occur in the ducts before stones are demonstrable, and strictures are often seen at cholangiography in the absence of stones. Conversely, stones are also found in the intrahepatic ducts when no significant narrowing of the ducts is discerned. In advanced cases, strictures are associated with extensive formation of stones, which can fill the ducts throughout the liver. Cisternal dilation of a duct may not be associated with very tight stenosis, and the cavernous ducts do not contain many stones; perhaps some ball-valve mechanism is responsible for these changes. In some patients with acute attacks, stones are not found, but infected, viscous bile permeates the entire biliary tree, which may represent the early stages of precipitation of bile before discernible stones are formed (see Chapter 7). Whatever the sequence of development, repeated or severe infection leads to transmural inflammation of the ducts and results in stenosis in the larger ducts, forming weblike strictures, and in the smaller peripheral ducts, showing more tubular narrowing. As a result of obstruction, together with parenchymal damage to the adjoining liver, the rest of the ducts dilate.
The calculi formed in RPC are pigmented bilirubinate stones (see Chapter 39). Infection in the bile duct changes the bile from a supersaturated solution to an insoluble precipitate. It is postulated that β-glucuronidase, derived from Clostridium perfringens and Escherichia coli, splits the bilirubin diglucuronide into free bilirubin, and the ionized unconjugated bilirubin together with ionic calcium precipitates to form insoluble calcium bilirubinate, which with time coagulates and consolidates into stones (Leung et al, 2001; Maki, 1966; Nakayama et al, 1980).
Although positive bile cultures are commonly obtained in patients with Western-type stones lodged in the CBD, when the stones are confined to the gallbladder, the incidence of infection is low. In contrast, regardless of whether the stones are located in the gallbladder or the CBD, the incidence of positive bile cultures in RPC is high (Suzuki et al, 1984; Tabata & Nakayama, 1981), a finding that favors infection as the primary step in the etiology of RPC. It also has been shown that in affected intrahepatic ducts, the number of mucous glands in the epithelial lining is increased (Nakanuma et al, 1988; Terada & Nakanuma, 1988). The integrated role of bacteria and mucus in the lithogenesis of hepatolithiasis was shown in a study by Zen and colleagues (2002), who found that lipopolysaccharide could induce overexpression of gel-forming apomucin (MUC2 and MUC5AC) in biliary epithelial cells via synthesis of tumor necrosis factor (TNF)–α and activation of protein kinase C. Mucin hypersecretion contributes to more stone formation by impeding bile flow and creating a nidus for pigment deposition (Sasaki et al, 1998). Augmented expression and secretion of trefoil factor family protein, a mucin-associated protein important for mucosal defense and repair, together with gel-forming apomucin may play a role in lithogenesis (Sasaki et al, 2004).
An association with infection by Clonorchis sinensis and Ascaris lumbricoides has been implicated in the past (Fung, 1961) and is still commonly regarded as causally significant (see Chapter 45; Rana et al, 2007). However, in countries where clonorchiasis is absent, such as the Philippines, RPC remains prevalent; and in Japan, where clonorchiasis is endemic, RPC is on the decline. Other evidence against clonorchiasis as a causal factor is that Clonorchis ova are isolated from the stools of only 25% of patients with RPC, and ascariasis is present in only 5% (Ong, 1962). It is indisputable that clonorchiasis is a serious infection that may cause structural changes in the intrahepatic and extrahepatic bile ducts (Hou, 1956). Although the cholangiographic changes of clonorchiasis are distinctly different from the changes of RPC (Choi et al, 1984), in that the terminal ducts are dilated rather than narrowed, the predominant and more severe changes are seen in the left duct. This occurrence corresponds to the distribution of RPC, a finding that still defies explanation.
Even if clonorchiasis and ascariasis are merely coincidental infections, they may become a nidus for stone formation (Teoh, 1963). Ascariasis probably plays no role in RPC except as a source of foreign bodies, and clonorchiasis may be a contributory factor in countries where RPC is endemic.
Pathology
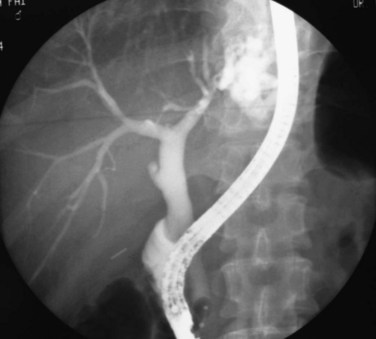
The primary pathologic changes are infection and fibrosis with strictures and stone formation in the bile ducts, with other changes being consequences of these main events. The consequences of repeated infection are progressive biliary epithelial and hepatocellular damage, as discussed previously. Suggested sequential changes in the bile ducts in RPC have been documented in detail by Lam and others (1978). These changes include loss of parallelism of duct walls, excessive branching, abrupt termination or “arrowhead” formation of smaller ducts, and development of strictures (Fig. 44.1).
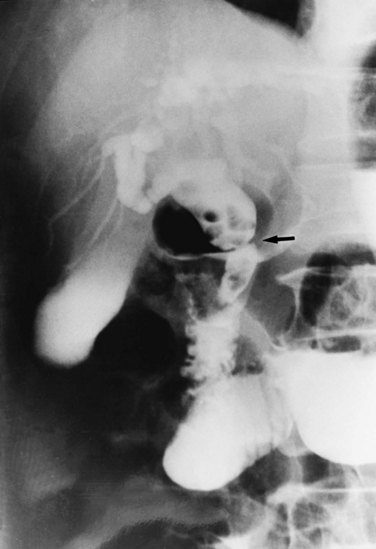
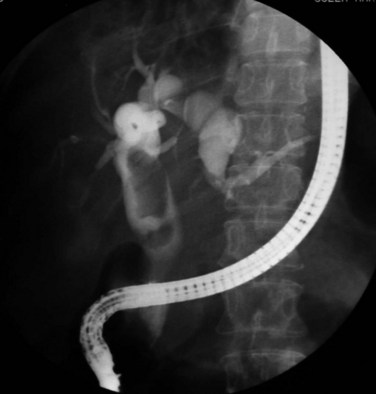
Strictures may be found anywhere in the biliary tree but are more common in the major hepatic duct branches, especially in the left liver and in the intrahepatic ducts. When in the extrahepatic ducts, strictures are weblike, situated toward the lower end; if the obstruction is severe, proximal dilation is marked (Fig. 44.2). Strictures in the hepatic ducts also extend over a short distance and are usually intrahepatic, but they may extend to the extrahepatic portion (Fig. 44.3). In the smaller intrahepatic ducts, the strictures are longer, and there may be a pattern of tubular narrowing over a length of duct (Fig. 44.4; also see Fig. 44.1).
The left duct is more frequently and severely affected than the right. Left duct involvement alone is found in 40% of cases of intrahepatic disease, right duct involvement alone is found in 20%, and involvement of both ducts is found in 40%. No satisfactory explanation has been offered for this finding, but it has been suggested that the left duct is more horizontal, and bile in the left duct may not drain as well as bile in the right duct. On the right side, one would expect the incidence of intrahepatic stones to be higher, if the right posterior hepatic duct joins the left hepatic duct at a sharp angle; however, a detailed study of the confluence patterns of segmental hepatic ducts did not show a causal relationship (Kitagawa et al, 2003).
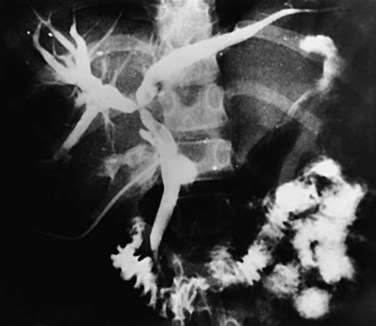
Proximal dilations behind the strictures are an expected secondary phenomenon. These dilations sometimes can be so large as to be called cisterns (Maki et al, 1964), and little liver parenchyma remains in such affected segments (Fig. 44.5). In these dilated ducts, relatively fewer stones are found (Fig. 44.6). Dilated segments taper toward the strictures, which are thick and fibrous; when operative plastic repair of such strictures is attempted, restenosis is common as a result of ongoing fibrotic changes in the diseased ductal tissues, and failure can be expected in most cases.
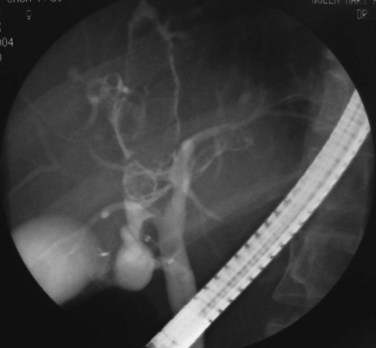
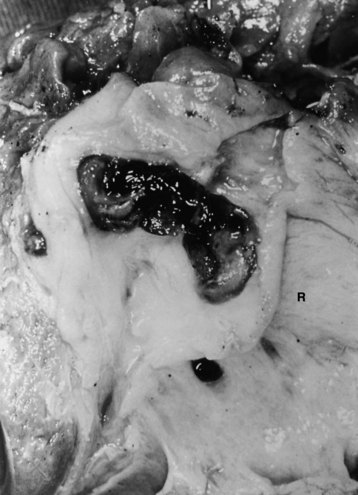
FIGURE 44.5 The right segmental duct is obstructed, and the proximal ductal system has become grossly dilated to form cisterns. Few stones are seen within the duct. The resected specimen is shown in Figure 44.6.

FIGURE 44.6 Resected specimen of the right segmental duct (endoscopic retrograde cholangiopancreatography is shown in Fig. 44.5) showing saccular enlargement of the intrahepatic ducts with stones. The surface is hemorrhagic, and the duct walls are thick.
The gallbladder is diseased in approximately 20% of patients with RPC, but in many patients with extensive ductal disease, the gallbladder is normal (see Figs. 44.2, 44.17A, and 44.19). When stones are found in the gallbladder, disease is invariably present elsewhere. In the acute attack, and when common duct obstruction is severe, the gallbladder may be grossly distended, and empyema, gangrene, or perforation may develop. When a normal gallbladder is left behind after drainage procedures to the common duct, the risk of developing a complication arising from the gallbladder that would require operation is small. When an operation is performed for patients with RPC, it is justified, although perhaps not recommended, to leave an apparently normal gallbladder when stones are found only in the bile duct.
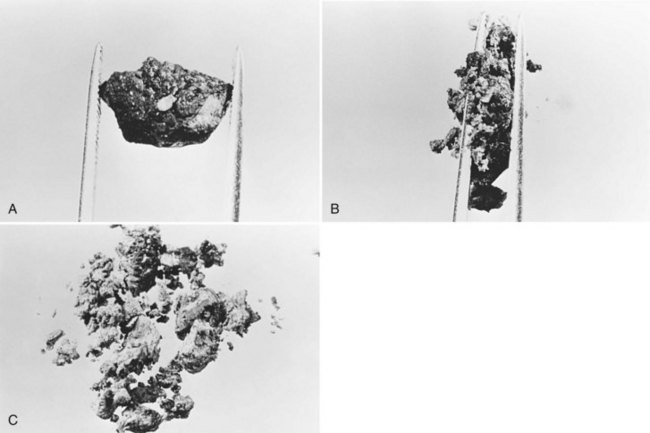
All stones recovered in RPC patients are bilirubinate stones: soft, pigmented, earthy stones that are very friable and crumble when pressed between the fingers. Application of forceps to these stones leads to fragmentation, and the small pieces usually left behind can be flushed out with saline (Fig. 44.7). The stones are irregular in shape and conform to the configuration of the bile duct in which they reside; when packed together, some may have facets. Their size varies from greater than 4 cm to almost microscopic, and in a single patient, a continuum in size may be seen, in contrast to the stepwise size change observed in Western-type mixed stones (Fig. 44.8).
In the fresh state, the stone surface is covered with mucus or a film of viscous bile. In some stones, the outer color may be almost black from prolonged exposure to bile; in others, it is orange or green. Flakes of more recently deposited bile debris are separated from the surface when gently scraped, exposing a lighter colored interior, which may appear laminated. Some stones show no organized structure and disintegrate with slight compression into irregular, powdery clumps. A nidus may sometimes be identified, and microscopic examination of this area may show dead parasites or clumps of bacteria or cells (Teoh, 1963).
At operation for an acute problem, the liver appears “cholangitic”: congested, bile stained, soft, and prone to bleeding easily. In the quiescent phase, avascular adhesions are found between the surface of the liver and the parietal peritoneum—evidence of previous, resolved acute episodes. In long-standing cases, the adhesions are dense and vascular and contain pockets of pus, which are due to rupture of cholangitic liver abscesses into the peritoneal cavity. Scars on the liver surface indicate previous attacks and dilated bile ducts and may appear prominently, especially from the undersurface of the left lobe (Fig. 44.9). When the left lobe is atrophic, compensatory hypertrophy of the right lobe is seen. Conversely, in the rare situation of severe right lobe disease, the left lobe may be massive, and the liver hilum anatomy may be grossly distorted (Fig. 44.10). Even when the external appearance is normal, intrahepatic disease may be extensive, and stones are easily palpable through the surface.
Biliary cirrhosis and liver failure are possible complications (Jeng et al, 1989) and usually follow long-standing severe disease that has failed to improve with multiple operations, some of which may be associated with stricture of the biliary-enteric anastomosis (see Chapters 29 and 42A). When cirrhosis has developed, portal hypertension and bleeding esophageal varices may ensue. Further corrective biliary surgery is feasible only after decompression by portosystemic shunting (see Chapter 76A, Chapter 76B, Chapter 76C, Chapter 76D, Chapter 76E ).
Stones at the lower end of the CBD may cause two additional complications in addition to biliary obstruction: choledochoduodenal fistula and acute pancreatitis. Choledochoduodenal fistula is not serious, but it may be confusing to the endoscopist and the radiologist. Acute pancreatitis is an important potential consequence of RPC. In Hong Kong, acute pancreatitis was once associated with RPC in approximately half of all patients (Ong et al, 1971), and in approximately 20% of patients with RPC, a high serum amylase level was recorded, although many cases were clinically silent.
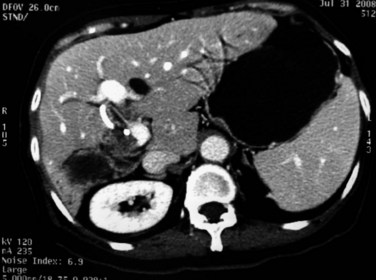
Although rare, abscesses in the left liver may rupture into the pericardial cavity and cause cardiac tamponade (Fan & Wong 1997). Abscesses in the right liver may rupture to form a pleurobiliary or bronchobiliary fistula (Wei et al, 1982). These abscesses also may bleed into the abscess cavity (Fig. 44.11) or bile duct (Joo et al, 2003), rupture into the abdominal cavity or into adjacent hollow viscera, or extend into the subphrenic or subhepatic spaces.
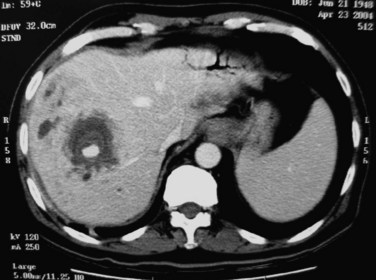
FIGURE 44.11 Computed tomographic scan of a patient with liver abscess that has produced a mycotic aneurysm.
A chronic abscess may be indistinguishable clinically, at operation or on contrast studies, from cholangiocarcinoma, and it may be identified as such only through detailed histologic examination after resection. As with hepatolithiasis, an increased incidence of cholangiocarcinoma owing to clonorchiasis has been noted (see Chapter 50A, Chapter 50B, Chapter 50C, Chapter 50D ; Hou, 1956; Ohta et al, 1984).
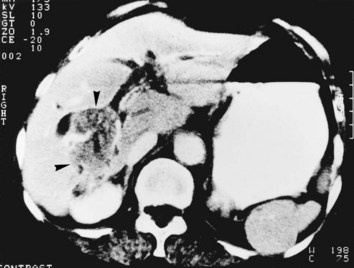
Whether cholangiocarcinoma is coincidental or etiologically related to RPC is controversial. The nearly constant presence of severe clonorchiasis in patients with cholangiocarcinoma supports a cause-and-effect relationship (Belamaric, 1973). Cholangiocarcinoma is found in 2% to 13% of patients with intrahepatic stones (Chen et al, 1989; Chu et al, 1997; Ohta et al, 1984, 1988). Autopsy studies suggest that recurrent cholangitis can induce progressive changes, leading to atypical epithelial hyperplasia and cholangiocarcinoma (Ohta et al, 1984). The tumor may take the form of a nodular or papillary growth, and stones may be found within the tumor mass or within the ductal lumen with tumorous invasion (Fig. 44.12). Cholangiocarcinoma should be suspected whenever a mass lesion is seen on imaging studies (Fig. 44.13); however, inflammatory pseudotumor (see Chapter 48) is also present in patients with RPC (Yoon et al, 1999). The imaging characteristics are nonspecific; only resection and pathologic examination can reliably differentiate the two conditions.
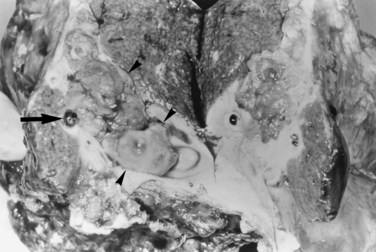
FIGURE 44.12 Right hepatectomy specimen of the same patient as in Figure 44.13, showing tumor mass (arrowheads) growing within the dilated bile duct; a black stone is seen within a branch of the bile duct (arrow).
Thrombophlebitis of major portal vein branches may develop when adjacent large biliary ducts are affected by RPC, causing extensive periductal inflammation (Fig. 44.14). The degreee of portal vein obstruction correlates well with the degree of liver atrophy (Kusano et al, 1991; see Chapter 5). When the hepatic veins become thrombosed, pulmonary emboli may develop; in rare instances, this can lead to pulmonary hypertension (Lai et al, 1968). Microscopically, the portal triads are infiltrated with inflammatory cells, and the cholangioles are filled with pus. In severe attacks, neutrophils are also seen in the sinusoids of the lobules, and adjacent hepatocytes undergo vacuolation. The larger bile ducts show acute inflammatory changes initially, but with repeated attacks, the ducts become thickened, surface mucosal lining is lost, and marked glandular proliferation advances into the thickened duct wall and beyond (Fig. 44.15). With repeated attacks, many of the glands undergo metaplasia, and fibrosis may extend far beyond the already thickened duct wall into the adjoining liver parenchyma, which undergoes degeneration and necrosis.
Clinical Features
In contrast to biliary calculous diseases seen in Western countries (see Chapter 30), RPC affects men and women equally, with a predilection for the lower socioeconomic classes. In Hong Kong today, with improved socioeconomic conditions, there are fewer new cases and fewer young patients with RPC. In a survey, the median age of patients with RPC was 59.5 years, and 56% had previous biliary operations for biliary stone disease (Liu et al, 1998).
The symptoms of RPC are not in themselves distinctive and are characteristic of acute cholangitis: pain, fever, and jaundice (Charcot’s triad; see Chapter 43). The pain is right hypochondrial or epigastric, and it may be distending, sharp, gnawing, or cutting, with frequent radiation to the back. It is constant, seldom colicky, and lasts for hours. Nausea is common, but vomiting is unusual. If the body temperature is elevated, septicemia or liver abscess must be suspected; the temperature chart often shows spikes rather than a continuous fever. Jaundice is seldom marked and may be just clinically perceptible, indicating incomplete obstruction. Pruritus is rarely a complaint, and the patient does not note pale stools. More typically, the patient is aware of the passage of tea-colored urine.
Should the abdominal signs deteriorate, indicating worsening peritonitis, or if generalized peritonitis is present, emergency operation or nonoperative intervention is mandatory. In elderly patients, abdominal signs may be minimal, even in septicemic patients, and reliance on physical findings alone may delay a decision to operate until the patient is in shock. Even when shock is present, there still may be a reluctance to operate on these elderly patients for lack of convincing abdominal signs. A transient increase in blood pressure in a patient with acute cholangitis may be a prelude to shock and must be regarded as a sign of impending deterioration rather than a positive response to treatment. In between attacks, there are few if any significant clinical features. Recent weight loss in elderly patients known to have RPC should raise the suspicion of development of cholangiocarcinoma, and it should also be suspected during follow-up when a patient’s serum alkaline phosphatase increases markedly (Kim et al, 2003), or when intrahepatic stones involving both lobes have not been completely cleared in previous operations (Jan et al, 1996).
Investigations
Imaging studies are important for the diagnosis of the disease, evaluation of the extent of involvement, and formulation of treatment plans for eradication of stones and strictures. Ultrasonography (US), computed tomography (CT), cholangiography, and magnetic resonance imaging (MRI) are complementary to each other in achieving such goals (see Chapters 13, 16, and 17).
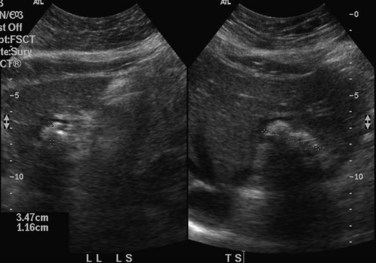
When applied optimally, US diagnoses the size of the common and intrahepatic ducts and the location of stones. It also shows liver abscesses, biloma, or tumor if any of these are present. Color Doppler US is useful in studying portal vein hemodynamics. Intrahepatic stones are readily identified if they cast sonic shadows (Fig. 44.16), but some stones found in RPC are isoechoic and may be isoechoic with respect to the surrounding tissue (Federle et al, 1982). This fact, combined with the propensity of these stones to form biliary casts, may lead to failure of US to identify intrahepatic stones in some cases (Chau et al, 1987). Another deficiency of US in the imaging of this disease is related to pneumobilia, which may produce highly reflective echoes and acoustic shadowing that simulates stones (Federle et al, 1982). Pneumobilia is a common finding in patients with RPC who have undergone biliary-enteric drainage procedures. In 30% of patients with RPC, prominent periportal echogenicity is found (Chau et al, 1987). These changes could represent pericholangitis and periportal fibrous thickening found in advanced stages of RPC, a finding that should prompt the ultrasonographer to search for other evidence of RPC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree