Chapter 50D Cancer of the bile ducts
Interventional techniques in hilar and intrahepatic biliary structures
Overview
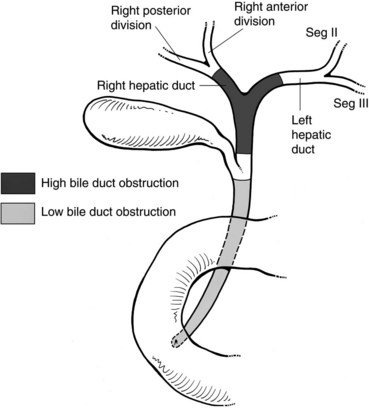
Malignant disease resulting in high bile duct obstruction (Fig. 50D.1)—that is, obstruction above the junction with the cystic duct—is not uncommon. Although frequently related to primary tumors of the biliary tree (see Chapters 49, 50A, and 50B), hilar obstruction, and even intraductal tumor, can be seen with other common malignancies such as breast, pancreatic, and colorectal cancers. Significant technical progress has occurred in both endoscopic and percutaneous biliary drainage, allowing safer palliative treatment of patients with such obstructions. Because these patients are often asymptomatic at presentation, the goals of treatment should be clearly defined prior to committing the patient to even a minimally invasive procedure. First and foremost, whether the patient is a surgical candidate should be determined. With the exception of patients who are clearly in a palliative situation, we prefer to discuss these patients in a multidisciplinary group with hepatobiliary surgeons, interventional radiologists, oncologists, and gastroenterologists to outline a plan of treatment. A thorough understanding of this plan and of the patient’s prognosis facilitates the development of a strategy for drainage. Accepted indications for palliative biliary drainage in these patients include intractable pruritus, cholangitis, the need to restore liver function to allow for administration of chemotherapeutic agents with biliary metabolism/excretion, access for intraluminal brachytherapy or choledochoscopy, and diversion for bile leak. Given the availability of high-quality magnetic resonance cholangiopancreatography (MRCP), direct cholangiography as a diagnostic tool is rarely warranted (see Chapters 17 and 18; Choi et al, 2008; Park et al, 2008).
Indications For Biliary Drainage
Neither hyperbilirubinemia alone nor the computed tomographic (CT) or ultrasound finding of dilated bile ducts is an indication for biliary drainage. Pruritus, cholangitis, and the need to lower the bilirubin to administer certain chemotherapeutic agents, on the other hand, are all accepted indications for biliary drainage. Patients who have undergone biliary-enteric bypass as part of curative resection for a benign or malignant lesion may develop postoperative bile leaks that require drainage for diversion. In some cases, access to the biliary tree may be undertaken as a method of delivering local treatment for primary bile duct cancer, such as brachytherapy or photodynamic therapy. Many physicians have the impression that patients feel better and have improved performance status after relief of jaundice; however, this has not been confirmed in clinical studies. Indeed, in a prospective trial, we have shown that in patients with malignant biliary obstruction, percutaneous drainage does not significantly improve quality of life (QOL), except for improving pruritus (Robson et al, 2007). Controversy remains in regard to the role of biliary drainage prior to surgery (Johnson & Ahrendt, 2006; Mezhir et al, 2009; Wang et al, 2008).
Endoscopic Versus Percutaneous Drainage (See Chapter 18, Chapter 27, Chapter 28 )
Patients with low bile duct obstruction are typically treated endoscopically. Patients with high bile duct obstruction, particularly when the obstruction extends above the hilus, have traditionally been treated percutaneously. This is because the success rate of percutaneous drainage has been higher, and the complication rate lower, when compared with endoscopic methods (Rerknimitr et al, 2004). This perspective is evolving as endoscope technology becomes more advanced, and endoscopists become better trained and more experienced in wire-guided procedures and have better guidewires and stents with which to work.
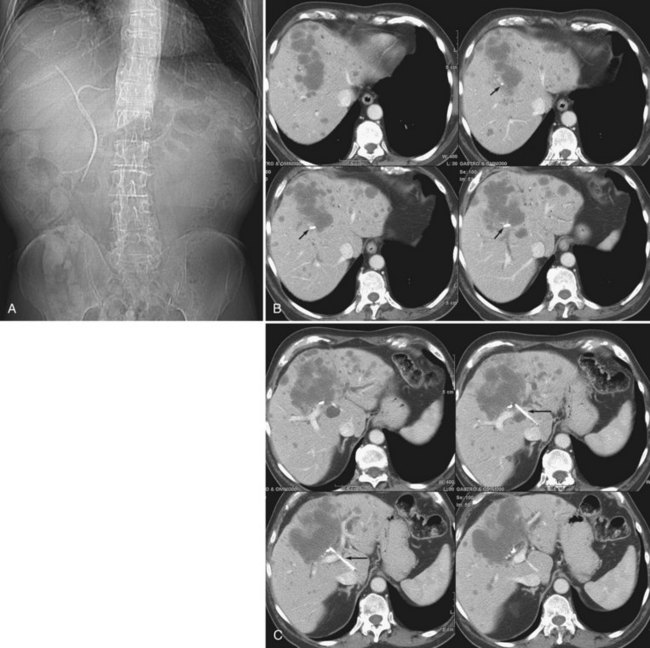
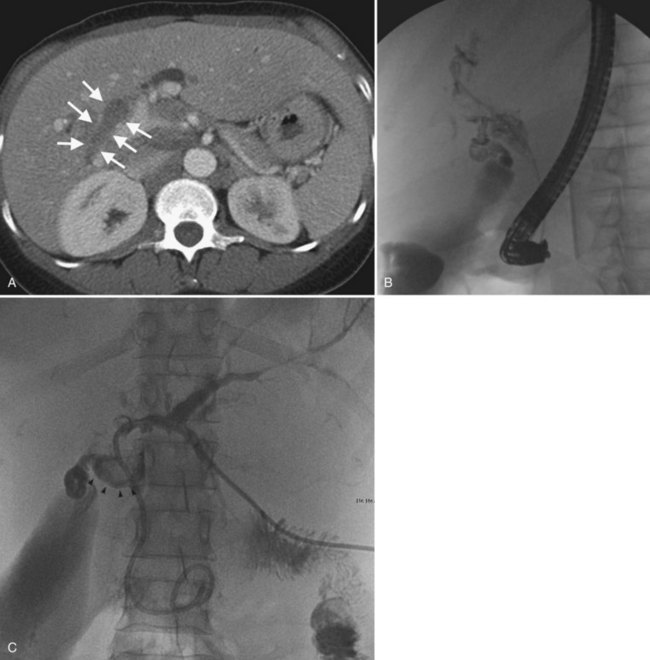
The two biggest drawbacks to endoscopic drainage for high bile duct obstruction have been the lack of ability to reliably target a specific area of the liver for drainage and the risk of contamination, by retrograde injection of contrast, of parts of the biliary tree that will not be drained. It is not uncommon to see a patient who is thought to have undergone successful endoscopic stenting of the right and left liver only to find, upon repeat imaging, that is not the case at all (Fig. 50D.2). Despite this, endoscopic management is indicated in some cases of high bile duct obstruction. This is particularly so when the risk of the patient having a permanent exteriorized catheter is high or when a patient has a higher risk of contaminating the entire biliary tree if approached percutaneously.
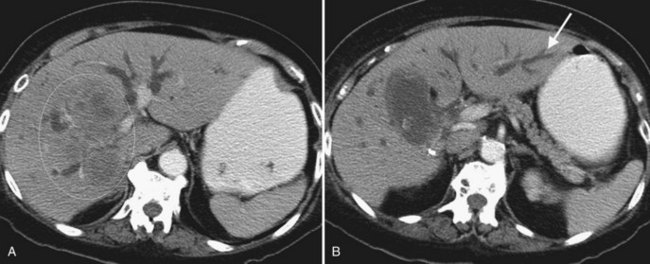
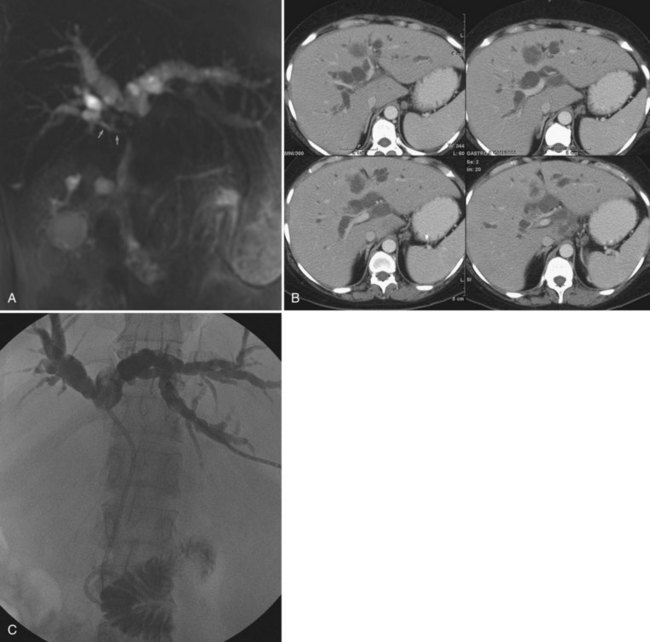
One exciting new endoscopic approach is the placement of a transgastric segment III stent using endoscopic ultrasound. This procedure is considered when a patient is best treated by draining the left lateral segment, and the percutaneous approach is technically difficult, or where the risk of contaminating the right-sided bile ducts is thought to be high with or without being able to cross the obstruction. This is particulary indicated when draining the right liver would be of no clinical benefit, either because of replacement of right liver by tumor or occlusion of the right portal vein (Fig. 50D.3). When technically feasible, this procedure can be gratifying for the patient, with relief of pruritus and lowering of bilirubin without the added burden of a percutaneous catheter. Endoscopic drainage is almost never indicated in patients with papillary intraductal tumor because it can easily grow into a metallic stent and result in early stent occlusion. Intraductal tumor can be seen from cancers other than papillary cholangiocarcinoma. When intraductal tumor occurs in the setting of metastatic colorectal cancer, it is usually the direct extension of a parenchymal metastasis into the duct. Patients with intraductal tumor often require permanent indwelling catheter drainage. The indwelling multiple sidehole catheter allows bile to drain around the intraductal tumor, and it can be changed every 10 to 12 weeks to maintain catheter patency (Fig. 50D.4). Having a realistic idea of what is feasible endoscopically and percutaneously is very important when making the initial decision regarding who will treat the patient. This depends on a thorough understanding of the goal of treatment as well as knowledge of the skill level of the interventional radiologists and endoscopists available to care for the patient.
Preprocedure Preparation
Imaging
The importance of excellent preprocedure imaging in patients with bile duct obstruction cannot be overemphasized. This imaging should include at a minimum a contrast-enhanced CT scan of the abdomen. Ultrasound is often used to establish the presence of dilated bile ducts, identify the level of obstruction, evaluate portal vein patency, and demonstrate intraductal tumor, but ultrasound is not adequate for drainage planning. Although MRCP provides an excellent three-dimensional (3D) rendering of the obstructed bile ducts and is excellent at delineating intraductal abnormalities and depicting isolated bile duct (Fig. 50D.5), it excludes certain things that can serve as targeting aides at the time of drainage, such as surgical clips, dystrophic calcifications, and bone landmarks. CT scans performed on multislice scanners in a picture archiving and communication system (PACS) environment allow the development of a 3D understanding of the anatomy while identifying relevant landmarks that can be used for targeting, and it also demonstrates other structures that should be avoided, such as liver lesions and bowel. Of course, as with MRI, one may also identify ascites, hepatic atrophy, and portal vein occlusion or encasement. All of this information is important in making prognostic assessments and in determining the best approach for drainage.
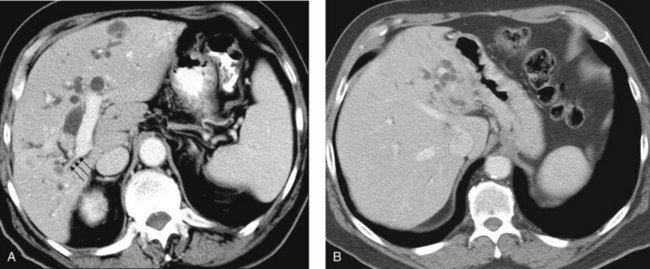
When planning drainage in patients with high bile duct obstruction, it is important to determine the amount of functional parenchyma that can be drained. Functional parenchyma is the part of the liver that is not replaced by tumor, and it has an intact portal venous supply. If 75% of the hepatic parenchyma is replaced by tumor, even if it were possible to drain the entire biliary tree, it is unlikely that normal hepatic function or normalization of serum bilirubin level would be restored. The portal vein provides the trophic blood supply to the liver, and occlusion results in atrophy of the affected segment or segments, particularly when the ipsilateral bile duct is also occluded (see Chapter 5; Hann et al, 1996). Atrophy may be recognized by the diminutive size of the involved part of the liver, accompanied by crowding of the bile ducts (Fig. 50D.6; see also Chapter 50B and Figs. 50B.6 and 50B.7). When portal vein occlusion causes atrophy, drainage of the atrophied liver does not improve liver function. In addition, it is frequently difficult to cross out of the obstructed/atrophied portion of the liver. The end result is an external drainage catheter that provides no clinical benefit to the patient but degrades QOL.
Central tumors frequently obstruct the right and left biliary tree, isolating them from one another. Obstruction may extend even higher, isolating the ductal system at the sectoral, segmental, or subsegmental level. When drainage is undertaken for relief of pruritus, draining even a segment of the liver may result in relief of symptoms (Abraham et al, 2002; Van Laethem et al, 2003). When isolation is present, and drainage is undertaken to lower the serum bilirubin, it is important to estimate how much functional parenchyma can be drained with one catheter. Functional liver parenchyma is defined as hepatic parenchyma with intact portal venous inflow and hepatic venous drainage. Analogous to a surgeon resecting no more than 75% of the liver to ensure an adequate liver remnant, it seems that at least 30% of functional hepatic parenchyma must be drained to improve liver function for chemotherapy, returning the serum bilirubin to normal or near-normal levels. In some cases, this goal may be impossible to achieve, unless more than one drainage catheter or stent is placed. Recognition of this allows for informing both the patient and referring physician about the potential need for more than one catheter or stent.
Cholangitis is rarely the primary indication for drainage in patients with malignant bile duct obstruction, except in patients who have undergone instrumentation and in those who have reason to have a contaminated biliary tree by virtue of previous biliary-enteric bypass, cross ampullary stenting, or sphincterotomy (Ozden et al, 2005). When draining a hemiliver or a segment, either percutaneously or endoscopically, it is possible to contaminate functionally “isolated” parts of the biliary tree. In this case, subsequent cholangitis may drive the placement of additional catheters. Widespread contamination of a severely isolated biliary tree with drainage of only part of the obstructed system can result in chronic recurrent cholangitis. Whether induced by endoscopic or percutaneous means, this situation can be difficult to manage and should be anticipated and avoided if possible.