Surgical resection of renal cell carcinoma remains the mainstay for the management of patients who suffer from this disease. Five percent to 10% of renal cell carcinomas develop a tumor thrombus that propagates into the renal vein or the inferior vena cava. Radical nephrectomy and inferior vena cava thrombectomy can provide longstanding survival rates comparable to those for tumors confined to the renal parenchyma. In general the surgical approach is dictated by the cephalad extension of tumor thrombus. This article reviews the authors’ experience with 243 patients who suffered from renal cell carcinoma with extension into the venous system with specific reference to the surgical techniques and the long-term outcomes.
Renal cell carcinoma (RCC) has been called the “internists’ tumor” for many years because of the myriad of vague symptoms associated with this malignancy. Patients historically presented with metastatic disease at the time of their initial diagnosis. At the present time, approximately 70% of the patients who have RCC are diagnosed as a result of enhanced imaging capabilities. This early diagnosis has produced a stage migration because many RCCs are discovered incidentally. Kidney cancer is the third most common urologic malignancy in the United States, representing 3.5% of the newly diagnosed cancers in the United States, and accounts for 2.3% of cancer-related deaths. During the past 20 years, the incidence of RCC has been increasing worldwide at a rate of 2.5% every year. This increased incidence is a result of increased abdominal imaging and an aging population. In addition, RCC is a highly vascular malignancy with a tendency to invade the venous system and create a tumor thrombus either in the renal vein or the inferior vena cava (IVC). An estimated 4% to 10% of RCCs have a tumor thrombus present in the venous circulation, specifically the renal vein and IVC, and a subpopulation of 1% has extension into the right atrium. Despite advances in radiation, chemotherapy, and immunotherapy the reference standard for RCC with tumor thrombus remains surgical resection. Several contemporary series have demonstrated 5-year survival rates of up to 60% in the absence of metastatic disease in patients who have venous tumor thrombus treated with radical nephrectomy and tumor thrombectomy.
Clinical presentation
The advent of cross-sectional imaging for the work-up of abdominal pain has resulted in an increase of RCC prevalence. A number of patients who have venous tumor thrombus can be asymptomatic depending on the level of the tumor thrombus and the extent of occlusion of the IVC. Significant venous congestion as a result of caval intrusion can present with varying symptoms, including significant lower extremity edema, varicocele formation, proteinuria, caput medusae, and even pulmonary emboli. If the tumor extends above the level of the hepatic veins, Budd-Chiari syndrome may result from obstruction of the major hepatic veins, resulting in a triad of hepatomegaly, abdominal fullness/pain, and ascites. The resulting varices produce massive collaterals with associated impaired hepatic function and portal hypertension. Additional symptoms of RCC include flank discomfort, hematuria, and constitutional changes (fever, weight loss, fatigue). These constitutional symptoms usually indicate the presence of metastatic disease with an overall poor prognosis.
Diagnostic imaging and preoperative evaluation
All patients who have renal masses must have imaging studies, including chest radiographs and bone scans when appropriate, to rule out metastatic disease. In patients who have venous extension, additional studies are necessary to define the extent of the tumor thrombus. For this subset of patients the authors’ preferred imaging modality is MRI, specifically magnetic resonance venography, in combination with CT studies and three-dimensional reformatted images ( Fig. 1 ). Tumor at or above the level of the diaphragm requires transesophageal echocardiography and may necessitate angiography to delineate the extent of the tumor thrombus. At the present time positron emission tomography (PET) scans have a limited diagnostic role; however, this modality is being evaluated at the Lahey Clinic as part of the preoperative evaluation and postoperative follow-up. The authors have noted that PET CT has demonstrated lesions in the liver that have not been detected on ordinary CT or MRI ( Fig. 2 ).
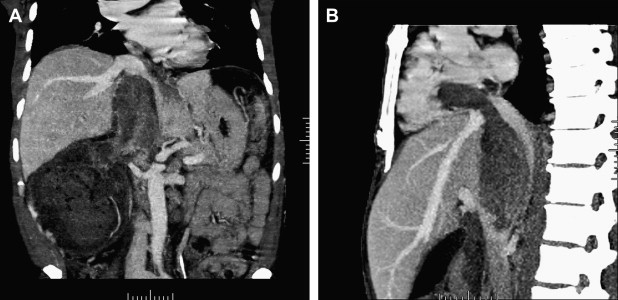
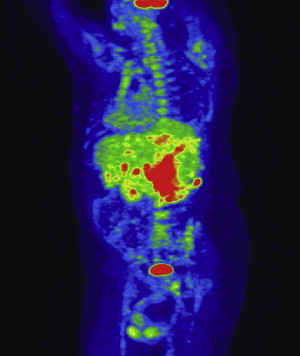
A report from Zini and colleagues suggests that preoperative measurements of the renal vein and IVC diameter with associated tumor thrombus correlate with the rate of renal ostial wall invasion and may serve as another prognostic indicator.
The importance of preoperative imaging for surgical planning cannot be overemphasized. Tumor thrombus extending to the level of the hepatic veins or higher may require cardiopulmonary bypass and circulatory arrest to provide insurance against excessive blood loss. Patients slated for cardiopulmonary bypass and circulatory arrest should have a cardiac evaluation, which may include stress testing or a coronary angiogram. If significant coronary artery disease is discovered, it may be treated with either a stent or bypass grafting. In five of the authors’ patients bypass grafting was performed concomitantly with the radical nephrectomy and IVC thrombectomy. Bland thrombus often can be distinguished from tumor thrombus during the preoperative evaluation, and anticoagulation therapy or placement of an IVC filter should be considered to limit further propagation and the possibility of a pulmonary embolus. Transesophageal echocardiography identifies the presence of tumor thrombus in the right atrium and is an important adjunctive intraoperative diagnostic modality.
Diagnostic imaging and preoperative evaluation
All patients who have renal masses must have imaging studies, including chest radiographs and bone scans when appropriate, to rule out metastatic disease. In patients who have venous extension, additional studies are necessary to define the extent of the tumor thrombus. For this subset of patients the authors’ preferred imaging modality is MRI, specifically magnetic resonance venography, in combination with CT studies and three-dimensional reformatted images ( Fig. 1 ). Tumor at or above the level of the diaphragm requires transesophageal echocardiography and may necessitate angiography to delineate the extent of the tumor thrombus. At the present time positron emission tomography (PET) scans have a limited diagnostic role; however, this modality is being evaluated at the Lahey Clinic as part of the preoperative evaluation and postoperative follow-up. The authors have noted that PET CT has demonstrated lesions in the liver that have not been detected on ordinary CT or MRI ( Fig. 2 ).
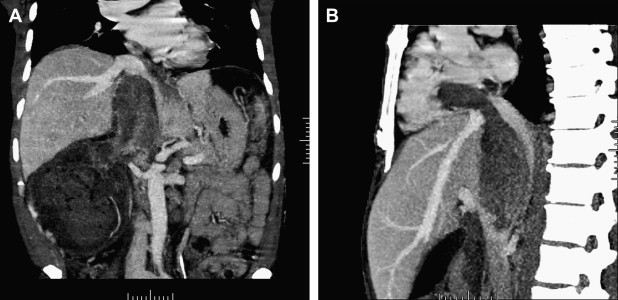
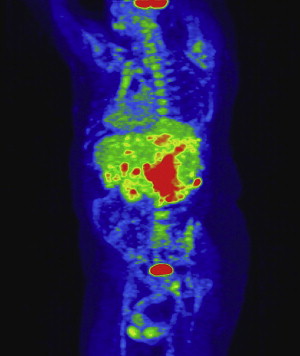
A report from Zini and colleagues suggests that preoperative measurements of the renal vein and IVC diameter with associated tumor thrombus correlate with the rate of renal ostial wall invasion and may serve as another prognostic indicator.
The importance of preoperative imaging for surgical planning cannot be overemphasized. Tumor thrombus extending to the level of the hepatic veins or higher may require cardiopulmonary bypass and circulatory arrest to provide insurance against excessive blood loss. Patients slated for cardiopulmonary bypass and circulatory arrest should have a cardiac evaluation, which may include stress testing or a coronary angiogram. If significant coronary artery disease is discovered, it may be treated with either a stent or bypass grafting. In five of the authors’ patients bypass grafting was performed concomitantly with the radical nephrectomy and IVC thrombectomy. Bland thrombus often can be distinguished from tumor thrombus during the preoperative evaluation, and anticoagulation therapy or placement of an IVC filter should be considered to limit further propagation and the possibility of a pulmonary embolus. Transesophageal echocardiography identifies the presence of tumor thrombus in the right atrium and is an important adjunctive intraoperative diagnostic modality.
Renal angioinfarction
Although only one prospective trial of preoperative angioinfarction is available to validate its use, the authors find that preoperative renal artery embolization is an important adjunctive tool in the treatment for advanced RCC, including patients who have venous tumor involvement. Preoperative renal angioinfarction facilitates the dissection of the renal tumor as a result of local tissue edema from hypoxia and tissue necrosis. In addition, it potentially may decrease the extent of the tumor thrombus while minimizing intraoperative blood loss associated with extensive venous collaterals. Renal angioinfarction also allows the surgeon to ligate or transect the renal vein before controlling or occluding the renal artery. Clinicians must be aware of the postinfarction syndrome caused by innate and humoral immune responses to the infracted kidney. This syndrome is characterized by chills, fevers, flank pain, malaise, hematuria, transient hypertension, and hyponatremia, all of which are self limiting.
Clinical staging and prognostics factors
There have been numerous proposals for staging RCCs and venous invasion. The current TNM staging system ( Box 1 ) designates tumor thrombus in the renal vein up to the diaphragm as T3b. Numerous retrospective studies at the Lahey Clinic and elsewhere advocate revision based on difference in survival when only the renal vein is involved. Literature, however, supports the current classification scheme, leaving the debate open for further discussion. In addition to the level of the tumor thrombus, a variety of centers are exploring additional prognostic factors for a revision of the current staging. The UCLA Integrated Staging System incorporates TNM stage, Fuhrman tumor grade, and the Eastern Cooperative Oncology Group performance status. Kattan and colleagues have developed a nomogram based on the TNM stage, patient symptoms, tumor size, grade, and vascular invasion or tumor necrosis. The SSIGN (stage, size, grade necrosis) model from the Mayo Clinic also has been evaluated in more than 1800 patients.
T: Primary tumor
Tx: Primary tumor cannot be assessed
T0: No evidence of primary tumor
T1: Tumor <7 cm in diameter, limited to kidney
T1a: Tumor 0–4 cm in greatest diameter, confined to kidney
T1b: Tumor 4–7 cm in greatest diameter, confined to kidney
T2: Tumor > 7 cm in greatest diameter, confined to kidney
T3: Tumor extends into major veins or invades adrenal gland or perinephric tissues but not beyond Gerota’s fascia
T3a: Tumor directly invades adrenal gland or perirenal and/or renal sinus fat but not beyond Gerota’s fascia
T3b: Tumor grossly extends into the renal vein or its segmental (ie, muscle-containing) branches or into the vena cava below the diaphragm
T3c: Tumor grossly extends into the vena cava above the diaphragm or invades the wall of the vena cava
T4: Tumor invades beyond Gerota’s fascia
N: Regional lymph nodes
NX: Regional lymph nodes cannot be assessed
N0: No regional lymph node metastasis
N1: Metastasis in a single regional lymph node
N2: Metastasis in more than one regional lymph node
M: Distant metastases
MX: Metastases cannot be assessed
M0: No distant metastases
M1: Distant metastases
Surgical treatment strategies
This discussion of surgical planning covers the following levels of tumor thrombus: renal vein, infrahepatic, retrohepatic, suprahepatic, supradiaphragmatic, and intra-atrial. Aggressive surgical management (radical nephrectomy, IVC thrombectomy, lymph node dissection, and potential metastectomy) remains the primary treatment modality, with the level of the tumor thrombus dictating the surgical approach. Tumors involving the caval venous system represent one of the most technically challenging and rewarding procedures for urologists because the 5-year survival rates, even in the face of intra-atrial tumor thrombus, are comparable to those for lesions confined to the kidney. A stepwise approach is discussed for each level of vein involvement focusing on techniques the authors have found successful in treating more 243 patients over a 30-year period ( Fig. 3 ). Additional techniques used by other urologic surgeons also are discussed in conjunction with specific scenarios.
Renal vein involvement
The invasion of the tumor thrombus at the level of the renal vein often can be approached using the principles of traditional radical nephrectomy first described by Robson and colleagues in 1969. The kidney and the great vessels can be exposed, mobilized, and controlled using a thoracoabdominal incision ( Fig. 4 ). In general a ninth or tenth intercostal incision is preferred. Significant venous collaterals can develop in the setting of venous tumor thrombus, particularly the lumbar drainage system. After ligation of the renal artery, the tumor thrombus is palpated gently to ensure that no further extension into the vena cava is present. A Satinsky clamp then is placed at the level of the renal vein ostium, sparing any lumbar tributaries. A circumferential incision is made at the level of the renal vein ostium. The caval defect is closed with running 4-0 polypropylene sutures, with caution taken to avoid constricting the diameter of the vena cava. With the venous system and arterial system ligated, a standard nephrectomy is performed with or without a lymph node dissection for staging purposes. In rare instances it is possible to spare the adrenal gland in lower pole tumors.
Suprarenal (infrahepatic tumor thrombus)
The authors categorize the presence of tumor thrombus below the level of the liver edge as infrahepatic. It often is possible to resect these lesions without bypass, because caval wall resection is rarely necessary, and bleeding can be controlled. The initial portion of this operation is to control the IVC with limited manipulation to prevent tumor embolus. The vena cava is dissected anteriorly and mobilized so that a Rummel tourniquet can be placed above and below the tumor thrombus and around each renal vein. Transesophageal echocardiography is performed to rule out propagation of the tumor thrombus. A chevron incision is made from the tip of the eleventh rib to the tip of the contralateral eleventh rib. The aorta and vena cava are exposed, and dissection is continued to allow placement of the Rummel tourniquets. As mentioned previously, large upper pole tumors also can be approached via a thoracoabdominal incision. The renal artery, associated lumbar and minor hepatic veins, and the contralateral renal vein are isolated and are dissected circumferentially. In many instances renal angioinfarction may produce an inflammatory response that precludes arterial mobilization; in this instance the authors defer ligation of the renal artery until the tumor thrombectomy has been completed. After the Rummel tourniquets are applied as described previously, a longitudinal cavatomy is made, and the thrombus is freed from the caval wall to the level of the renal vein ostium ( Fig. 5 ) The IVC then is gently flushed with heparinized saline and is evaluated for residual fragments. The cavotomy is closed with continuous 4-0 polypropylene sutures. The infrarenal clamp is released initially to purge the system and to limit the chance of embolus. Radical nephrectomy and/or lymph node dissection is performed after closure of the vena cava has been completed.
Suprarenal thrombus (retrohepatic and supradiaphragmatic)
Surgical removal of RCC with suprarenal retrohepatic tumor thrombus can be accomplished with a variety of surgical techniques with equivalent oncologic outcomes. The authors’ experience with hypothermic circulatory arrest and cardiopulmonary bypass is one of the largest series published to date with outcomes comparable to those of contemporary colleagues. The authors have described techniques of vascular and liver mobilization that have provided excellent exposure to the retrohepatic portion of the IVC ( Fig. 6 ). After a chevron incision is made and the absence of metastatic disease is confirmed, the anterior surface of the IVC is identified and is palpated gently to confirm the cephalad extent of the tumor thrombus. In some cases the thrombus can be gently milked caudally for clamp placement. This procedure must be performed with caution to prevent embolization of the thrombus. Traditionally the duodenum is kocherized, and the Langenbuch maneuver is used to mobilize the liver cephalad and to the left, thus exposing the retrohepatic portion of the IVC ( Fig. 7 ). The kidney is mobilized completely with the exception of the renal vein and associated tumor thrombus ( Fig. 8 ). The IVC then is mobilized completely from the renal vein to the cephalad extent of the tumor thrombus, ensuring retroperitoneal hemostasis before heparinization and cardiopulmonary bypass. The right subclavian artery and superior vena cava are cannulated, and cardiopulmonary bypass is initiated ( Fig. 9 ). Thiopental and methylprednisolone are given as the core temperature is cooled to 18° to 20°C and the patient is exsanguinated. Patients can withstand up to 40 minutes of circulatory arrest time without neurologic insult and may be exposed to extended periods with the use of retrograde cerebral perfusion. A right atriotomy provides distal control of the tumor thrombus, minimizing the risk of embolization ( Fig. 10 ). An anterior cavotomy is made from the level of the renal vein to the level of the hepatic veins, and the thrombus is extracted with the patient in Trendelenberg’s position and using positive-pressure respiration ( Fig. 11 ). In some situations a Fogarty catheter is passed into the atrium and/or hepatic veins to retrieve portions of the tumor thrombus. The authors use venacavascopy with a flexible cystoscope to ensure that tumor thrombus removal is complete. Efforts should be made to remove the kidney and thrombus as one specimen ( Fig. 12 ). The advantage of bypass is an essentially bloodless operative field, but the authors recognize and accept the complications of bypass, including coagulopathy and the potential for neurologic complications.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








