Matthew A. Bailey, David G. Shirley, Robert J. Unwin
Renal Physiology
The prime function of the kidney is to maintain a stable milieu intérieur by the selective retention or elimination of water, electrolytes, and other solutes. This is achieved by three processes: (1) filtration of circulating blood from the glomerulus to form an ultrafiltrate of plasma in the urinary space (Bowman space), (2) selective reabsorption (from tubular fluid to blood) across the cells lining the renal tubule, and (3) selective secretion (from peritubular capillary blood to tubular fluid).
Glomerular Structure and Ultrastructure
The process of urine formation begins by the production of an ultrafiltrate of plasma. Chapter 1 describes glomerular anatomy and ultrastructure, so this discussion provides only the essentials for understanding how the ultrafiltrate is formed. The pathway for ultrafiltration of plasma from the glomerulus to Bowman space consists of the fenestrated capillary endothelium, the capillary basement membrane, and the visceral epithelial cell layer (podocytes) of Bowman capsule; the podocytes have large cell bodies and make contact with the basement membrane only by cytoplasmic foot processes. Mesangial cells, which fill the spaces between capillaries, have contractile properties and are capable of altering the capillary surface area available for filtration.
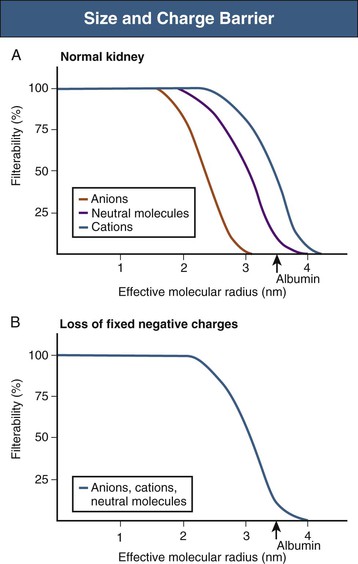
Filtration is determined principally by the molecular size and shape of the solute and, to a much lesser extent, by its charge. The size cutoff is not absolute; resistance to filtration begins at an effective molecular radius of slightly less than 2 nm, whereas substances with an effective radius exceeding about 4 nm are not filtered at all. The fenestrations between capillary endothelial cells have a diameter of 50 to 100 nm. The podocyte foot processes have gaps with a diameter of 30 to 40 nm, although these filtration slits are bridged by the slit diaphragms, which are themselves penetrated by small pores. The slit diaphragms likely constitute the main filtration barrier, although both the endothelium (by preventing the passage of blood cells) and the basement membrane contribute.1 The “subpodocyte space” also provides an additional and variable resistance to glomerular filtration.2 Furthermore, the podocytes and the endothelial cells are covered by a glycocalyx composed of negatively charged glycoproteins, glycosaminoglycans, and proteoglycans, and the basement membrane is rich in heparan sulfate proteoglycans. This accumulation of fixed negative charges further restricts the filtration of large, negatively charged ions, mainly proteins (Fig. 2-1). Thus, with an effective radius (3.6 nm) permitting significant filtration, albumin is normally almost completely excluded. If these fixed negative charges are lost, as in some forms of early or mild glomerular disease (e.g., minimal change disease), albumin filterability increases and proteinuria results. Although it has been proposed that albumin is normally filtered and then almost completely reabsorbed along the proximal tubule, the evidence is controversial. The glomerular barrier is usually considered as a passive unidirectional filter. However, recent studies indicate that filtration pressure generates a potential difference between the glomerular capillaries and Bowman space. Although small in magnitude, this potential difference may help to clear the filter continuously, driving negatively charged proteins such as albumin out of the slit diaphragm and back into the blood.3
Glomerular Filtration Rate
At the level of the single glomerulus, the driving force for glomerular filtration (the net ultrafiltration pressure) is determined by the net hydrostatic and oncotic (colloid osmotic) pressure gradients between glomerular plasma and the filtrate in Bowman space. The single-nephron glomerular filtration rate (SNGFR) is determined by the product of the net ultrafiltration pressure and the ultrafiltration coefficient; the latter being a composite of the surface area available for filtration and the hydraulic conductivity of the glomerular membranes. Therefore, the single-nephron glomerular filtration rate is as follows:
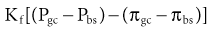
where Kf is the ultrafiltration coefficient, Pgc is glomerular capillary hydrostatic pressure (~45 mm Hg), Pbs is Bowman space hydrostatic pressure (~10 mm Hg), πgc is glomerular capillary oncotic pressure (~25 mm Hg), and πbs is Bowman space oncotic pressure (0 mm Hg).
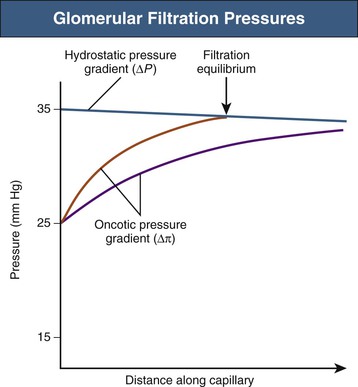
Net ultrafiltration pressure is about 10 mm Hg at the afferent end of the capillary tuft. As filtration of plasma from blood proceeds along the glomerular capillaries, proteins are concentrated and the glomerular capillary oncotic pressure (πgc) increases. Theoretically, toward the efferent end of a glomerular capillary, πgc may equal the net hydrostatic pressure gradient, at which point ultrafiltration pressure would fall to zero: filtration equilibrium in the human kidney is approached, but rarely (if ever) achieved (Fig. 2-2).
The total glomerular filtration rate (GFR) is the sum of the SNGFRs of the functioning nephrons in each kidney. The normal range for GFR is wide but typically cited at about 120 ml/min per 1.73 m2 surface area. GFR can be measured with renal clearance techniques. The renal clearance of any substance not metabolized by the kidneys is the volume of plasma required to provide that amount of the substance excreted in the urine per unit time. This is a virtual volume that can be expressed mathematically as follows:
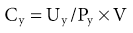
where Cy is the renal clearance of y; Uy and Py is the concentration of y in the urine and plasma, respectively; and V is the urine flow rate. If a substance is freely filtered by the glomerulus and is not reabsorbed or secreted by the tubule, its renal clearance equals GFR; that is, renal clearance measures the volume of plasma filtered through the glomeruli per unit time. The various methods for measuring GFR and their pitfalls are discussed in Chapter 3.
Measurement of Renal Plasma Flow
The use of the clearance technique and the availability of substances that undergo both glomerular filtration and virtually complete (or effective) tubular secretion have made it possible to measure renal plasma flow (RPF; typically ~650 ml/min). Para-aminohippuric acid (PAH, hippurate) is an organic acid that is filtered by the glomerulus and actively secreted by the proximal tubule through organic anion transporters in the cell membranes. The amount of PAH found in the urine is the sum of that filtered plus that secreted. PAH clearance is a robust marker of RPF when the plasma concentration is less than 10 mg/dl, because most of the PAH reaching the peritubular capillaries is cleared by tubular secretion. Under these circumstances, little PAH appears in renal venous plasma and the amount found in the final urine approximates that delivered to the kidneys in the plasma. Therefore:
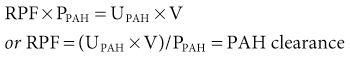
where UPAH and PPAH are the concentrations of PAH in the urine and plasma, respectively, and V is the urine flow rate. Renal blood flow (RBF) can be calculated as follows:
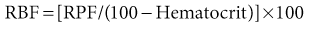
Typically, RBF is about 1200 ml/min.
The most important limitation of this method is the renal extraction of PAH, which is always less than 100%. At high plasma concentrations, greater than 10 to 15 mg/dl, the tubular transport proteins become saturated, the fractional tubular secretion of PAH declines, and considerable amounts of PAH appear in the renal veins. Under these circumstances, PAH clearance significantly underestimates RPF. In patients with liver or renal failure, the production of toxins and weak organic acids can interfere with PAH secretion or cause tubular damage, leading to inhibition of PAH transport. Certain drugs, such as probenecid, are organic acids and compete with PAH for tubular secretion, thereby reducing PAH clearance. Also, the expression of transport proteins that mediate PAH secretion is hormonally regulated, and the clearance of PAH can therefore change independent of true RPF.
Autoregulation of Renal Blood Flow and Glomerular Filtration Rate
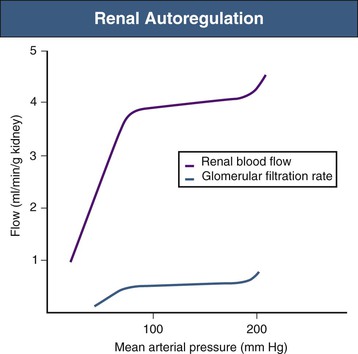
Although acute physiologic variations in arterial blood pressure inevitably cause corresponding changes in RBF and GFR, these are short-lived because compensatory mechanisms return both RBF and GFR toward normal within a few seconds.4 This is the phenomenon of autoregulation (Fig. 2-3). Autoregulation is achieved primarily at the level of the afferent arterioles and believed to result from a combination of the following two mechanisms:
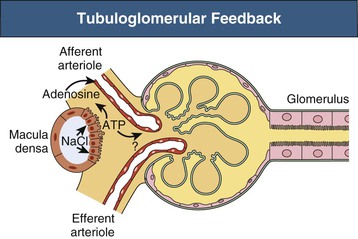
Because these mechanisms restore both RBF and Pgc toward normal, the initial change in GFR is also reversed. The TGF system is possible because of the juxtaglomerular apparatus (see Chapter 1), which consists of the macula densa region of each nephron and the adjacent glomerulus and afferent and efferent arterioles (Fig. 2-4). The primary mediator of TGF is adenosine triphosphate (ATP). Increased NaCl delivery to the macula densa leads to increased NaCl uptake by these cells, which triggers ATP release into the surrounding extracellular space.5 It is thought that ATP has a direct vasoconstrictor effect, acting on P2X1 purinoceptors on afferent arteriolar cells; although evidence also indicates that nucleotidases present in this region degrade ATP to adenosine, which, acting on afferent arteriolar A1 receptors, can also cause vasoconstriction.6 The sensitivity of TGF is modulated by locally produced angiotensin II, nitric oxide, and certain eicosanoids (see later discussion).
The TGF regulation of filtration rate may be more complex than typically described, with evidence for regulatory crosstalk between the distal nephron and the vasculature at sites beyond the macula densa,7 as well as for synchronization of blood flow across networks of nephrons in response to changes in sodium delivery.8
Despite renal autoregulation, a number of extrinsic factors (nervous and humoral) can alter renal hemodynamics. Independent or unequal changes in the resistance of afferent and efferent glomerular arterioles, together with alterations in Kf (thought to result largely from mesangial cell contraction/relaxation), can result in disproportionate, or even contrasting, changes in RBF and GFR. In addition, changes in regional vascular resistance can alter the distribution of blood flow within the kidney. For example, medullary vasoconstriction may affect whole-kidney blood flow because blood can be diverted through the cortex: nevertheless, this renders the medulla hypoxic and vulnerable to ischemic injury.9 Figure 2-5 indicates how, in principle, changes in afferent and efferent arteriolar resistance can affect net ultrafiltration. Table 2-1 outlines vasoactive factors that alter renal hemodynamics (see Integrated Control of Renal Function). In addition, damage to the renal afferent arteriole, as in patients with hypertension and progressive kidney disease, may also interfere with renal autoregulatory mechanisms.
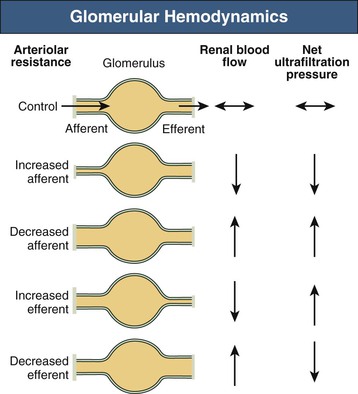
Table 2-1
Physiologic and pharmacologic influences on glomerular hemodynamics.
The overall effect on glomerular filtration rate (GFR) will depend on renal blood flow, net ultrafiltration pressure, and the ultrafiltration coefficient (Kf), which is controlled by mesangial cell contraction and relaxation. The effects shown are those seen when the agents are applied (or inhibited) in isolation; the actual changes that occur are dose dependent and are modulated by other agents. ACE, Angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; ANP, atrial natriuretic peptide; NSAIDs, nonsteroidal anti-inflammatory drugs: PGE2/PGI2, prostaglandins E2 and I2.
| Physiologic and Pharmacologic Influences on Glomerular Hemodynamics | ||||||
| Arteriolar Resistance | ||||||
| Afferent | Efferent | Renal Blood Flow | Net Ultrafiltration Pressure | Kf | GFR | |
| Renal sympathetic nerves | ↑↑ | ↑ | ↓ | ↓ | ↓ | ↓ |
| Epinephrine | ↑ | ↑ | ↓ | → | ? | ↓ |
| Adenosine | ↑ | → | ↓ | ↓ | ? | ↓ |
| Cyclosporine | ↑ | → | ↓ | ↓ | ? | ↓ |
| NSAIDs | ↑↑ | ↑ | ↓ | ↓ | ? | ↓ |
| Angiotensin II | ↑ | ↑↑ | ↓ | ↑ | ↓ | ↓→ |
| Endothelin-1 | ↑ | ↑↑ | ↓ | ↑ | ↓ | ↓ |
| High-protein diet | ↓ | → | ↑ | ↑ | → | ↑ |
| Nitric oxide | ↓ | ↓ | ↑ | ? | ↑ | ↑(?) |
| ANP (high dose) | ↓ | → | ↑ | ↑ | ↑ | ↑ |
| PGE2/PGI2 | ↓ | ↓(?) | ↑ | ↑ | ? | ↑ |
| Calcium channel blockers | ↓ | → | ↑ | ↑ | ? | ↑ |
| ACE inhibitors, ARBs | ↓ | ↓↓ | ↑ | ↓ | ↑ | ?* |
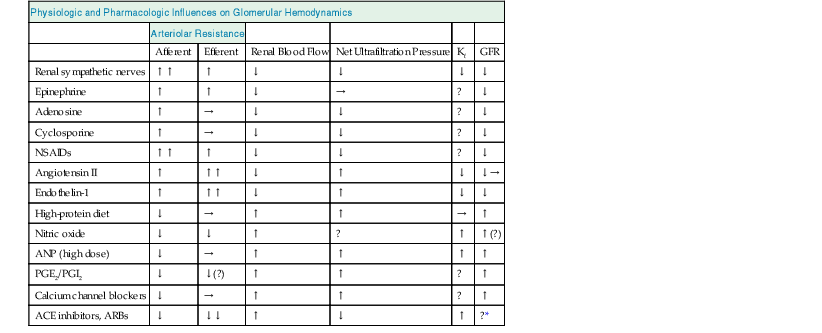
* In clinical practice, GFR is usually either decreased or unaffected.
Tubular Transport
Vectorial transport is net movement of substances from tubular fluid to blood (reabsorption), or vice versa (secretion). The cell membrane facing the tubular fluid (luminal or apical) must have different properties than the membrane facing the blood (peritubular or basolateral). Such epithelia are said to be “polarized,” thus allowing the net movement of substances across the cell (transcellular route). The tight junction, which is a contact point close to the apical side of adjacent cells, limits water and solute movement between cells (paracellular route).
Solute transport across cell membranes uses either passive or active mechanisms.
Passive Transport
Simple diffusion always occurs down an electrochemical gradient, which is a composite of the concentration gradient and the electrical gradient. With an undissociated molecule, only the concentration gradient is relevant, whereas for a charged ion, the electrical gradient must also be considered. Simple diffusion does not require a direct energy source, although an active transport process is usually necessary to establish the initial concentration and electrical gradients.
Facilitated diffusion (or carrier-mediated diffusion) depends on an interaction of the molecule or ion with a specific membrane carrier protein that facilitates its passage across the cell membrane’s lipid bilayer. In almost all cases of carrier-mediated transport in the kidney, two or more ions or molecules share the carrier; one moiety moves down its electrochemical gradient, while the other(s) move against the gradient.
Diffusion through a membrane channel (or pore) formed by specific integral membrane proteins is also a form of facilitated diffusion, because it allows charged and lipophobic molecules to pass through the membrane at a high rate.
Active Transport
Ion movement directly against an electrochemical gradient (“uphill”) requires a source of energy and is known as active transport. In cells, this energy is derived from ATP production and its hydrolysis. The most important active cell transport mechanism is the sodium pump, which extrudes sodium ions (Na+) from inside the cell in exchange for potassium ions (K+) from outside the cell.10 In the kidney, this process is confined to the basolateral membrane. The Na pump derives energy from the enzymatic hydrolysis of ATP and thus is more precisely termed Na+,K+-ATPase. It exchanges 3Na+ for 2K+ and is electrogenic because it extrudes a net positive charge from the cell; Na+,K+-ATPase is an example of a primary active transport mechanism. Other well-defined primary active transport processes in the kidney are the proton-secreting H+-ATPase, important in hydrogen ion secretion in the distal nephron, and the Ca2+-ATPase, partly responsible for calcium reabsorption.
Activity of the basolateral Na+,K+-ATPase underpins the operation of all the passive transport processes outlined earlier. It ensures that the intracellular Na+ concentration is kept low (10 to 20 mmol/l) and the K+ concentration high (~150 mmol/l), compared with their extracellular concentrations (~140 and 4 mmol/l, respectively). The pump-leak model of sodium transport uses the electrochemical gradient established and maintained by the Na pump to allow “leak” of Na+ into the cell through a variety of membrane transport proteins. These can be Na+ channels (in the distal nephron) or specific membrane carrier proteins that couple Na+ entry to the influx (symport or cotransport) or efflux (antiport or countertransport) of other molecules or ions. In various parts of the nephron, glucose, phosphate, amino acids, K+, and chloride ions (Cl−) can all be cotransported with Na+; moreover, H+ and Ca2+ can be countertransported against Na+ entry. In each case, the non-Na molecule or ion is transported against its electrochemical gradient, using energy derived from the “downhill” movement of Na+. Their ultimate dependence on the Na+,K+-ATPase makes them secondary active transport mechanisms.
Transport in Specific Nephron Segments
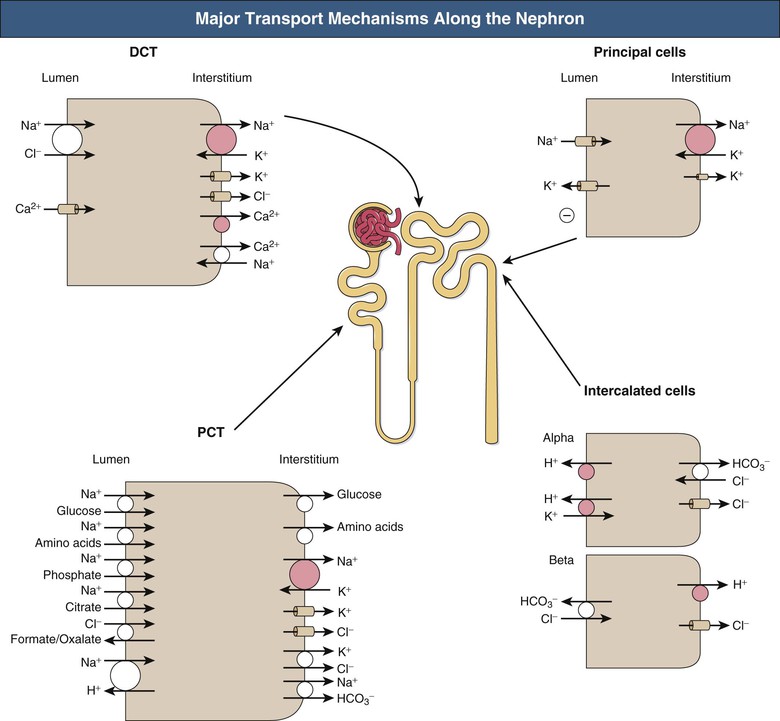
Given a typical GFR, approximately 180 liters of plasma (largely protein-free) is filtered each day, necessitating massive reabsorption by the whole nephron. Figure 2-6 shows the major transport mechanisms operating along the nephron (except the loop of Henle, dealt with separately).