Chris Baylis, John M. Davison
Renal Physiology in Normal Pregnancy
There are profound changes in renal function in normal pregnancy that lead to marked alterations from the nonpregnant physiologic norm. An appreciation and understanding of these alterations are essential to recognize both normal and compromised pregnancies.
Anatomy
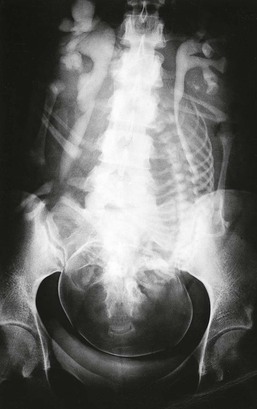
In normal pregnancy, there is a progressive increase in renal bipolar diameter, maximally 1 to 2 cm by 26 weeks’ gestation, and kidney volume enlarges by about 70% because of increments in both vascular and interstitial fluid compartments.1 The most striking anatomic change is dilation of the calyces, renal pelvis, and ureter (more prominent on right side), and by the third trimester, about 80% of women show evidence of hydronephrosis2 (Fig. 43-1). A consequence of the ureteral dilation is urinary stasis, which predisposes pregnant women with asymptomatic bacteriuria to development of symptomatic ascending infection (acute pyelonephritis). Rarely, the anatomic changes may be extreme and precipitate the overdistention syndrome, with massive dilation, recurrent severe flank pain, increasing serum creatinine, hypertension, or even reversible acute kidney injury.3
Systemic Hemodynamics
There are significant alterations in systemic hemodynamics in normal pregnancy. A plasma (and extracellular fluid) volume expansion occurs while red blood cell volume also increases, leading to a large increase in blood volume that correlates with clinical outcome and birth weight. Interestingly, subsequent pregnancies tend to be more successful than the first, with larger babies and greater plasma volume increases. Women with twins and triplets have proportionately greater increments, and those with poorly growing fetuses, as in preeclampsia or with a history of poor reproductive performance, have correspondingly poor plasma volume responses. The increase in plasma volume (maximum increase ~1.25 liters) takes place progressively up to 32 to 34 weeks, after which there is little further change. The plasma volume expansion has a hemodilutional effect, causing decreases in hematocrit: the physiologic anemia of normal pregnancy.4
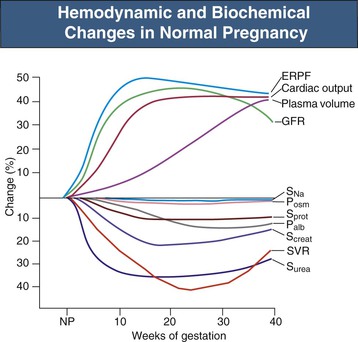
Cardiac output is significantly increased by the fifth gestational week, initially caused by a 10% to 20% increase in heart rate, with stroke volume increased by more than 20% the eighth week. Left atrial and left ventricular end-diastolic dimensions increase, suggesting an associated increase in venous return. Cardiac output increases of 40% to 50% are greatest by the 26th week, despite which systemic blood pressure (BP) substantially decreases in normal pregnancy (representative values are shown in Fig. 43-2 and Table 43-1).5 The physiologic decrease in BP results from a profound reduction in systemic vascular resistance (SVR) of unknown cause (maximal at 26 weeks), although the loss of responsiveness to vasoconstrictor agents (e.g., angiotensin II, arginine vasopressin) certainly contributes.6 Inhibition of angiogenic factors in preeclampsia causes vasoconstriction, suggesting that these factors, such as vascular endothelial growth factor (VEGF), may contribute importantly to normal gestational vasodilation through stimulation of endothelial nitric oxide and prostaglandins.7 The combination of increased cardiac output and peripheral vasodilation means that organ blood flow increases in pregnancy, with the most dramatic changes occurring in the kidney and skin circulation throughout gestation and in the uterus in the second part of the pregnancy.1 Left ventricular mass continues to increase up to 36 weeks, but thereafter, perhaps related to the increasing SVR toward term, systolic and diastolic cardiac function decrease, with increased ventricular wall stress, as evidenced by decrements in both myocardial contraction and relaxation capacity.8
Table 43-1
Changes in some common indices during pregnancy.
BP, Blood pressure; Pco2, carbon dioxide partial pressure.
| Changes in Some Common Indices During Pregnancy | ||
| Nonpregnant | Pregnant | |
| Hematocrit (%) | 41 | 33 |
| Serum protein (g/dl) | 7.0 | 6.0 |
| Plasma osmolality (mOsm/kg) | 285 | 275 |
| Serum sodium (mmol/l) | 140 | 135 |
| Serum creatinine (mg/dl, µmol/l) | 0.8 (73) | 0.5 (45) |
| Blood urea nitrogen (mg/dl) | 12.7 | 9.3 |
| Serum urea (mmol/l) | 4.5 | 3.3 |
| pH units | 7.40 | 7.44 |
| Arterial Pco2 (mm Hg) | 40 | 30 |
| Serum bicarbonate (mmol/l) | 25 | 20 |
| Serum uric acid (mg/dl, µmol/l) | 4.0 (240) | 3.2 (190) early 4.3 (260) late |
| Systolic BP (mm Hg) | 115 | 105 |
| Diastolic BP (mm Hg) | 70 | 60 |
(Mean values compiled from references 9-11, 19, and 36.)
In the third trimester, the enlarged uterus compresses surrounding tissues and can influence hemodynamic measurements, so that attention should be paid to maternal posture during hemodynamic monitoring. In the supine position, there is partial obstruction of the inferior vena cava and decreased venous return, reducing cardiac output and causing a decrease in BP, the supine hypotensive syndrome of pregnancy. It is important to be aware of these postural effects in measuring BP in late pregnancy.1
Renal Hemodynamics
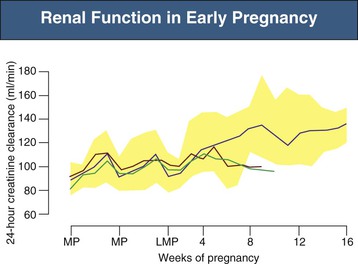
There are striking changes in renal hemodynamics in normal pregnancy, with an increase in glomerular filtration rate (GFR) and consequent decrease in serum creatinine detectable very early.1,9 The GFR increases about 25% by 4 weeks after the last menstrual period, and a robust early increase in GFR (see Fig. 43-2) is associated with a good obstetric outcome. Longitudinal studies in normal pregnant women show that GFR (measured by inulin or 24-hour creatinine clearance) increases by a maximum of about 50% by midpregnancy, which is maintained until the last few weeks of the pregnancy, when values begin to decrease, but remain above the nonpregnant level (Fig. 43-3).10 These marked increases in GFR mean that serum creatinine decreases to 0.4 to 0.5 mg/dl (36 to 45 µmol/l),11 and values considered normal for nonpregnant conditions, at 0.7 to 0.8 mg/dl (63 to 72 µmol/l), can be a cause for concern in normal pregnancy (see Table 43-1). In small women, however, whose total muscle mass may be quite low, significantly elevated serum creatinine levels may be absent, even in the presence of renal dysfunction. The increase in renal plasma flow (RPF) of about 60% is slightly more pronounced than the increase in GFR (see Fig. 43-2), so that the filtration fraction (FF) decreases (see later discussion). At the end of pregnancy, the RPF decreases proportionally more than the GFR, so that FF returns to the nonpregnant value.10,12
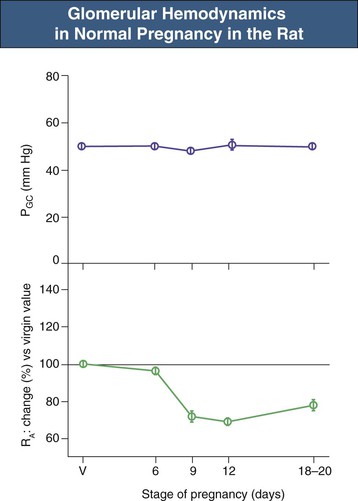
A similar pattern of renal hemodynamic change occurs during pregnancy in some animals, including the rat, in which GFR increases to a maximum of 30% to 40% above the “virgin” value by midterm, with a late return toward the nonpregnant value close to term (22 days). Glomerular micropuncture studies have shown that the increase in GFR is paralleled by increases in single-nephron GFR secondary to increased glomerular plasma flow.13 Because the preglomerular and postglomerular resistance vessels dilate in parallel, glomerular plasma flow increases without a change in glomerular BP. As shown in Figure 43-4, the glomerular capillary blood pressure (PGC) remains unchanged throughout the pregnancy despite marked alterations in preglomerular vascular resistance. Similar conclusions have been reached by an indirect modeling approach in normal pregnant women measuring whole-kidney GFR, RPF, and serum protein concentrations; in addition, polydisperse neutral dextran was infused for determination of dextran sieving curves.9,12 This approach allows (with certain reasonable assumptions) modeling of glomerular hemodynamics. In pregnant women, there is a decrease in serum protein concentration that contributes slightly to the increased GFR. As in the rat, the majority of the gestational increase in GFR in normal women is caused by increased RPF with no change in glomerular BP. The constancy of glomerular BP during sustained renal vasodilation has important implications for the long-term effects of pregnancy on renal function, as discussed later.
It is often assumed that a change in FF reflects a change in glomerular BP, but this is not always the case because FF is also determined by Kf, the product of water permeability of the glomerular wall and total glomerular capillary surface area.10 Both glomerular wall water permeability and filtration pressure are very high, and thus filtration proceeds rapidly. In some situations, not all the available filtration surface area is used, so that filtration ceases (because the driving pressure is exhausted) before the end of the glomerulus. This state is known as filtration pressure equilibrium. When plasma flow increases during filtration pressure equilibrium, a proportional increase in GFR occurs with no change in FF. This is seen in the normal pregnant rat.13 In contrast, FF decreases during normal pregnancy in women as RPF is increasing.10 This most likely reflects that humans are closer than rats to filtration pressure disequilibrium, a situation in which the entire glomerular capillary surface area available for filtration is used, leaving a positive driving pressure at the end of the glomerulus. At filtration pressure disequilibrium, GFR becomes less dependent on plasma flow; thus an increase in plasma flow (with no change in other determinants of filtration) leads to a disproportionately smaller increase in GFR, with a decrease in FF.14
Despite the prolonged renal vasodilation, the renal vasculature remains fully responsive to various stimuli during pregnancy. For example, in the rat, the intrinsic renal autoregulatory ability remains intact,15 although the myogenic response of the interlobular artery is blunted in normal rat pregnancy.16 The tubuloglomerular feedback component of renal autoregulation is reset to recognize the elevated GFR as normal in the pregnant rat,17 and a recent clinical study reports increased an increased renal artery resistive index during normal pregnancy, suggestive of maintained renal autoregulatory ability.1 Both pregnant rats and women exhibit a marked additional renal vasodilation in response to amino acid infusion,18,19 demonstrating substantial renal vasodilatory reserve in normal pregnancy. The cause of the gestational increase in GFR remains uncertain, although studies in the pseudopregnant rat have shown that the fetoplacental unit is not necessary,20 indicating that a maternal stimulus must initiate the gestational renal hemodynamic changes.
A number of vasoactive factors have been evaluated as possible mediators of the renal vasodilation,13 and although there are no clinical data, animal studies have implicated a role for nitric oxide (NO).21–23 A novel splice variant of the neuronal NO synthase is induced in the kidney cortex of the pregnant rat, which parallels the increased renal plasma flow and GFR.24 Increases in renal cortical antioxidant capacity may also contribute to enhanced renal NO levels in the normal pregnant rat.25 Studies have suggested a key role for the ovarian hormone relaxin, which may signal increased renal NO production in pregnancy, possibly through an endothelin type B receptor mechanism.26,27 The renal vasodilatory signal of pregnancy is remarkably robust because women with single kidneys (organ donors) and renal transplant recipients who have already undergone significant compensatory renal hypertrophy and vasodilation can produce further increases in RPF and GFR in pregnancy.9,10 In the human, the signal is not solely relaxin as there is still a gestational increase in GFR, although blunted, in infertile women who lack corpus luteum function and who conceive from ovum donation and assisted conception techniques.28
Furthermore, although relaxin levels do increase steeply early in pregnancy (peaking at 6 weeks, declining thereafter to 36 weeks, but still much higher than pre-pregnancy),1 there is no correlation between the levels and any hemodynamic parameter and no differences in levels between normal and preeclamptic pregnancies, despite substantial and persistent GFR abnormalities in the latter.29 Also, although relaxin may be one important renal vasodilator in pregnancy, complementary factors and changes likely permit concomitant GFR increments because to attain levels akin to early pregnancy, women infused with recombinant human relaxin significantly augment RPF but not GFR.30
Glomerular hypertension associated with renal vasodilation is considered a primary pathogenic stimulus to progressive renal injury in chronic kidney disease.31 As discussed, normal pregnancy is also a state of chronic renal vasodilation; however, glomerular BP remains normal. This may account for the findings that repetitive pregnancies in women and rats with normal renal function have no long-term adverse effects on glomerular function or structure.32
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree