Annabel C. Martin, Mark A. Brown
Renal Complications in Normal Pregnancy
Most women have normal pregnancies. The most common renal problem that arises in pregnancy in an otherwise well woman is urinary tract infection (UTI). Also, by far the most common de novo “renal disease” in the second half of pregnancy is preeclampsia, usually presenting with hypertension and proteinuria. Fortunately, acute kidney injury in pregnancy is rare in developed countries, but still carries a poor prognosis for this young age group.
Urinalysis and Microscopy
Many young women have their first urinalysis and urine microscopy performed during pregnancy, leading to the detection of hematuria, proteinuria, and pyuria, either related to or coincidental to the pregnancy.
Hematuria
Definition and Epidemiology
Microscopic hematuria is detected at some time during pregnancy in about 20% of women.1 Clinically significant microhematuria is defined as three or more red blood cells (RBCs) per high-power field on microscopic evaluation of urinary sediment from two of three properly collected (clean-catch, midstream) urine specimens,2 or as greater than 2500 RBCs/ml.3 Microhematuria disappears in about 75% of women after delivery, but secondary to glomerulonephritis, it persists throughout the pregnancy, and can be further investigated postpartum.
Etiology and Outcome
Dysmorphic microhematuria (defined by phase contrast microscopy) during pregnancy is most frequently caused by glomerulonephritis but is occasionally associated with preeclampsia. Isomorphic hematuria is more likely to be caused by bladder infection or bladder compression by the fetal head. Pregnant women with microhematuria showed no significant difference in gestational age at delivery, birth weight, gestational hypertension, or preeclampsia compared with controls.4
Macrohematuria (gross hematuria) in pregnancy is most often the result of vaginal bleeding or hemorrhagic bacterial cystitis. Other, less common causes include renal calculi, renal arteriovenous malformations, polycystic kidneys, and rarely, bladder or kidney neoplasms.
Differential Diagnosis
When microhematuria is found in the pregnant patient, a urine culture is required to exclude infection. If there is no proteinuria, and blood pressure (BP) and serum creatinine are normal, further investigations can be delayed until postpartum review, usually about 3 months, when microscopy to determine RBC morphology, antinuclear antibody testing, and renal ultrasound can be performed.
When significant numbers of dysmorphic urinary RBCs are present during pregnancy and BP is normal, the most likely cause is glomerular disease, either thin basement membrane nephropathy or IgA nephropathy. If microhematuria is associated with renal angle pain or renal colic, ultrasound will exclude the presence of stone in only two thirds of pregnant women with calculi and may demonstrate other abnormalities such as polycystic kidneys and in rare cases neoplasms. For persistent isomorphic microhematuria, we do not recommend cystoscopy during pregnancy. This often disappears spontaneously after pregnancy, and the likelihood of uroepithelial tumors is very low in this age group.
Treatment
For the pregnant patient with microhematuria, the treatment of associated UTI and calculi is discussed later. There is no specific treatment for glomerulonephritis during the pregnancy, provided renal function is normal and nephrotic syndrome is absent.
Proteinuria
The development of proteinuria during pregnancy warrants investigation and will most often be associated with preeclampsia. A condition known as gestational proteinuria may also develop, which has no adverse effects for fetus or mother.
Definition
Normal pregnancy is associated with an increase in protein excretion compared with the nonpregnant state. Some studies suggest this results from a combination of increased glomerular filtration rate and increased permeability of the glomerular basement membrane in normal pregnancy.5 Others have found 24-hour urinary albumin excretion similar in nonpregnant and normal pregnant women, with more than 20 mg/day considered abnormal; in such patients, increased total proteinuria must be caused by tubular proteinuria.
In contrast to abnormal total protein excretion defined as greater than 150 mg daily in nonpregnant women, proteinuria in pregnancy is generally defined as the excretion of more than 300 mg of total protein per 24 hours. However, more than 95% of pregnant women excrete less than 200 mg/day.6
Proteinuria is most frequently detected in pregnancy by dipstick urinalysis, but this method is notoriously unreliable, with a significant proportion of false-positive and false-negative results. Dipstick is generally reliable for confirming either the absence of proteinuria or the presence of true proteinuria, when dipstick readings are 3+ (>3 g/L) or 4+ (>20 g/L). At intermediate levels, false-positive rates are as high as 50%. Detection may be improved using an automated urinalysis device, thereby reducing observer error.7
The 24-hour urine collection remains the gold standard for quantitation but is often impractical when a quick assessment of proteinuria is required, as in preeclampsia. A reliable alternative is the protein-creatinine ratio; a ratio greater than 30 mg protein/mmol creatinine (~0.3 mg/mg) correlates reasonably well with protein excretion of more than 300 mg/24 h.8
Although use of the albumin-creatinine ratio may gain a greater role in pregnancy in the future, clinicians currently should continue to use the protein-creatinine ratio until further data are available.9
Differential Diagnosis
Proteinuria arising de novo in pregnancy should be quantitated and further investigated according to the clinical situation. The course of pregnancy in women with proteinuria present before pregnancy is discussed in Chapter 45.
Persistent proteinuria associated with preeclampsia usually arises in the second half of pregnancy, and generally but not always after the development of hypertension. Occasionally, proteinuria predates all other signs of preeclampsia. The severity of the proteinuria is not indicative of the severity of preeclampsia and should not be used to guide management. Furthermore, proteinuria may be absent in 10% of women with clinical and histologic manifestations of preeclampsia.10 In the absence of UTI or preeclampsia, isolated proteinuria in an otherwise asymptomatic pregnant woman usually reflects new-onset renal disease such as a primary glomerulonephritis. Alternatively, proteinuria may appear because pregnancy has unmasked renal disease secondary to systemic disorders, such as diabetes mellitus, systemic lupus erythematosus, or essential hypertension. In addition, superimposed preeclampsia occurs in 20% to 25% of women with chronic hypertension.11 In these patients, it often occurs earlier in gestation and may be severe.
We limit investigation of de novo non-preeclamptic, non-nephrotic proteinuria during pregnancy to a renal ultrasound and the measurement of serum creatinine, electrolytes, albumin, and antinuclear antibodies. Renal biopsy is not indicated. When underlying renal disease has been unmasked by pregnancy, appropriate investigations can usually be delayed until the postpartum period. The exceptions to this are women with nephrotic syndrome or renal impairment in whom fetal viability is not yet ensured and whose pregnancy needs to be continued (i.e., <24 weeks’ gestation). In these patients, full investigation should be undertaken quickly, usually including renal biopsy, to determine whether specific treatment (e.g., corticosteroids) may be of benefit.
Natural History
Proteinuria occurring as a complication of preeclampsia invariably resolves in the postpartum period, although this may take several months. Proteinuria in women with gestational proteinuria, an entity that describes new-onset proteinuria without features of preeclampsia but with intermediate changes in angiogenic factors (see later), also resolves postpartum. Tradition holds that in both cases, proteinuria disappears within 3 months postpartum, but that many cases take longer.12 In the absence of renal function or impaired function, we do not investigate non-nephrotic proteinuria until it persists after 12 months postpartum.
Treatment
For women with nephrotic syndrome, an inverse correlation exists between serum albumin and birth weight, although no studies have examined whether reduced fetal growth can be reversed by reducing proteinuria. Antiproteinuric therapy with angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are contraindicated in pregnancy because of their unwanted effects on the fetus.
Intravenous (IV) albumin has little role in managing nephrotic syndrome during pregnancy unless there is progressive deterioration in renal function. We recommend IV albumin infusion when serum creatinine rises above 100 µmol/l (1.2 mg/dl) with no other explanation, acknowledging that this may only provide a temporary benefit to allow the pregnancy to be prolonged. Diuretics and sodium restriction should be avoided unless the woman has severe, intractable edema; the concern is that diuretics will lead to a further reduction in plasma volume and reduced uteroplacental perfusion. The pregnant woman with nephrotic syndrome is at high risk of venous thromboembolism and should receive prophylactic heparin during pregnancy and until the nephrotic syndrome has resolved postpartum.
Pyuria
Isolated pyuria, in the absence of UTI, is common in normal pregnancy, usually from contamination by vaginal secretions. There is also an increased white blood cell excretion rate in normal pregnancy. It requires no further follow-up other than ensuring it disappears, usually by 3 months postpartum.
Urinary Tract Infection
Definitions
In asymptomatic bacteriuria (ASB), the urinary tract is colonized by a single species of bacteria in the absence of specific symptoms. Outside of pregnancy, ASB usually should not be treated with antibiotics. However, ASB can lead to serious complications in pregnancy, and there is a cost benefit from its screening and treatment.13 Although the gold standard for diagnosis is detection of more than 105 organisms per milliliter, without epithelial cells, in a midstream urine specimen on two or more occasions, or from a single suprapubic aspiration,14 in practice such bacteriuria is usually detected by a single routine bacterial culture early in pregnancy. As many as 1% to 2% of pregnancies are complicated by acute bacterial cystitis, defined as an acute bacterial UTI accompanied by symptoms such as frequency, dysuria, or strangury. Although more than 105 organisms/ml defines ASB, as few as102 organisms/ml is sufficient to diagnose cystitis if accompanied by pyuria and characteristic symptoms.15 In acute pyelonephritis there is UTI, generally with more than 105 organisms/ml, in association with parenchymal renal infection, usually diagnosed clinically by fever and loin pain and sometimes features of systemic sepsis.
Epidemiology
Asymptomatic bacteriuria affects 2% to 10% of all pregnant women. The prevalence is higher in women from lower socioeconomic groups and increases with age, parity, coexistent genital tract infection, and sickle cell trait. ASB is also more common in women with urinary tract abnormalities such as reflux nephropathy and neurogenic bladder, in diabetic patients, and in women with several previous UTIs. The highest risk of infection is thought to be from the 9th to the 17th week of gestation, although this may simply reflect the stage at which routine screening usually takes place.
The overall incidence of acute pyelonephritis in pregnancy is approximately 1%, but it may occur in up to 30% among women with ASB. It is thought that about 70% of women who develop acute pyelonephritis have preceding covert bacteriuria, but this is difficult to prove. With treatment of ASB, it has been estimated that the incidence of pyelonephritis would be reduced by more than 80%.15 When possible, urethral catheters should be avoided, even in women having cesarean section, because the incidence of UTI in these women is twice that of those not catheterized.
Pathogenesis
Certain host characteristics may increase the risk for UTI or pyelonephritis (see Chapter 53). Women, such as those who do not express the antibody to the O antigen of Escherichia coli, may have ASB that predates pregnancy (i.e., are chronically colonized). Pregnancy is a state of relative urinary tract stasis; the calyces, pelves, and ureters dilate, particularly on the right, and this contributes to ASB developing into ascending acute pyelonephritis in pregnant women.
The most common mechanism of infection is through the urethra from perineal bacteria. Box 44-1 lists common organisms causing UTI in pregnancy. Some strains of E. coli are particularly virulent and are associated with both ASB and pyelonephritis. They possess P fimbriae, which enable the bacteria to attach themselves to the uroepithelial cells with pili, allowing them to ascend the urinary tract from the perineum.
Clinical Manifestations
Asymptomatic Bacteruria
A meta-analysis indicated that untreated ASB during pregnancy significantly increases rates of low birth weight (LBW) and preterm delivery.16 However, it is still unclear whether ASB is an independent risk factor for LBW or whether its association with low socioeconomic status is the predictor for LBW. The mechanism by which UTI may cause premature labor is not yet fully understood, but proinflammatory cytokines secreted in response to bacterial endotoxins likely initiate labor in these patients.
Most maternity units operate a policy of screening all pregnant women on at least one occasion, whether by dipstick urinalysis for leukocytes/nitrites or by direct urine culture. Both dipstick and urine culture appear cost-effective compared with no screening when the prevalence of ASB is 2% to 6%. As stated, isolated pyuria on dipstick testing is very common in normal pregnancy, and we recommend screening by primary urine culture rather than the dipstick method.
Women who have threatened premature labor should have further urine cultures even without symptoms suggestive of UTI.
Pyelonephritis
Pyelonephritis most often presents between 20 and 28 weeks of gestation with malaise, fever, loin pain, and rigors. Not all women will have had lower urinary tract symptoms, and pyelonephritis can also manifest in pregnancy as acute abdominal pain, or may be detected only after presentation with premature labor. Pyelonephritis is more common in pregnant women with urologic abnormalities or diabetes and more often affects the right kidney, probably because the ureter is generally more dilated on that side.
The diagnosis of pyelonephritis is usually made on clinical grounds. Definitive diagnosis requires positive urine culture, but this may take about 2 days, and treatment should not be delayed. E. coli is the most common infecting organism (>85% of cultures).
Bacteremia is a common and usually transient complication of pyelonephritis. Occasionally, however, women become septicemic and may develop endotoxemia with shock, with sequelae including respiratory failure, disseminated intravascular coagulation, and acute kidney injury. Pyonephrosis and perinephric abscess are rare complications but should be suspected when treatment fails.
Without treatment, the complications of acute pyelonephritis during pregnancy can be severe, probably more so than in nonpregnant women. In the preantibiotic era, maternal mortality was 3% to 4%. Currently, death from pyelonephritis is rare in developed countries but still occurs.
Treatment
Asymptomatic Bacteruria
Antibiotic treatment of ASB significantly reduces the incidence of pyelonephritis in pregnancy. A systematic review also suggests treatment results in a significant reduction in the risk of preterm delivery.18
In choosing treatment, the clinician must consider the safety of the antibiotic in pregnancy. In most women, treatment with cephalexin, amoxicillin–clavulanic acid, or nitrofurantoin is first-line therapy. Overall, trials demonstrate that longer duration of treatment is likely to be more effective than single-dose therapy, although data are limited.18 Until evidence from new trials is available, a 4- to 7-day treatment regimen is recommended, although some authorities allow for a 3-day rather than 7-day course of antibiotics.17
Without treatment, ASB will persist in 80% of women, and even with treatment, 20% will still have ASB. Those with persistent colonization are difficult to treat, with eradication achieved in only 40% after a second course of antibiotics. Where eradication is not achieved, we recommend prophylactic antibiotics, usually cephalexin (250 mg at night), throughout pregnancy to prevent pyelonephritis and its consequences; however, no studies specifically address this situation. The incidence of pyelonephritis after effective treatment of ASB is reduced from about 30% to 3%, comparing favorably with a 1% prevalence of pyelonephritis for the overall pregnant population.
Cystitis
Treatment of cystitis at the first presentation should be for 5 days with an appropriate antibiotic. As for ASB, it is important to obtain a follow-up urine culture to be certain infection has been eradicated.
Pyelonephritis
It is usual practice to admit pregnant women with pyelonephritis to the hospital, although a trial has reported successful outpatient management for milder cases.18 Treatment may occasionally require resuscitation with IV fluids, but usually a short course of IV antibiotics followed by oral antibiotics, once the woman is afebrile, is adequate therapy.
It is desirable to choose an antibiotic that produces a high blood level that will concentrate in the renal parenchyma, usually a cephalosporin as first-line treatment. An aminoglycoside is a useful adjunct in more severe cases, used for 24 to 48 hours while awaiting urine cultures, provided maternal renal function is satisfactory. The risk of irreversible fetal ototoxicity precludes prolonged use, and aminoglycosides are not recommended in the first trimester. The full duration of treatment is generally at least 2 weeks, and it is imperative to repeat urine culture 1 week after treatment to ensure eradication.
Renal ultrasound is generally not indicated during an initial infection, because urinary tract dilation is still likely to be present, and it is impossible to distinguish this from significant urinary tract obstruction. However, if infection persists, ultrasound is indicated to help exclude pyonephrosis, perinephric abscess, and renal calculi. If pyelonephritis persists despite adequate antibiotic therapy and urinary tract dilation is confirmed, percutaneous nephrostomy should be performed under ultrasound guidance. In our experience, this is rarely necessary and should be a last resort in management of these cases, but nephrostomy is the only way to be certain urinary tract obstruction and pyonephrosis have been properly treated.
Clinicians also should remain alert for premature labor in the presence of pyelonephritis and institute appropriate treatment while aggressively treating the infection. When more than two UTIs have occurred in pregnancy, prophylaxis is indicated until delivery with either nitrofurantoin (50 mg) or cephalexin (250 mg) at night. Table 44-1 outlines some antibiotic regimens suitable for use in pregnancy.
Table 44-1
Antibiotic regimens for treatment for urinary tract infections in pregnancy.
| Antibiotic Regimens for Treatment of Urinary Tract Infections in Pregnancy | ||
| Antibiotic | Dose | Duration |
| Acute Cystitis | ||
| Amoxicillin | 500 mg three times daily | 3-7 days |
| Nitrofurantoin | 100 mg twice daily | 3-7 days |
| Cephalexin | 500 mg two or three times daily | 3-7 days |
| Asymptomatic Bacteriuria | ||
| Cephalexin | 500 mg three times daily | 3 days |
| Amoxicillin | 500 mg three times daily | 3 days |
| Amoxicillin–clavulanic acid | 500 mg three times daily | 3 days |
| Nitrofurantoin | 50 mg four times daily | 3 days |
| Fosfomycin | 3 g single dose | |
| Recurrent Bacteriuria or Cystitis | ||
| Cephalexin | 250 mg nighttime (or postcoital) | |
| Nitrofurantoin | 50 mg nighttime (or postcoital) | |
| Amoxicillin | 250 mg nighttime (or postcoital) | |
| Pyelonephritis (Initial IV Therapy) | ||
| Ceftriaxone | 1 g daily | |
| Ampicillin (with gentamicin) | 1 g every 6 hours | |
| Gentamicin | 3 mg/kg daily | |
| Ticarcillin | 3.2 g every 8 hours | |
| Piperacillin | 4 g every 8 hours | |
Renal Calculi
Epidemiology
Despite the physiologic state of pregnancy being an ideal environment for renal stone formation, the incidence of renal calculi remains similar in pregnant and nonpregnant women, in the range of 0.03% to 1%.
Pathogenesis
The majority of stones are formed from calcium oxalate and calcium phosphate. Struvite stones are the next most common, usually when the urinary tract is infected with organisms such as Proteus spp. Small proportions of renal stones are formed from uric acid or cystine. As discussed, pregnancy is a physiologic state of relative urinary stasis, as well as of increased calcium and uric acid excretion. The incidence of renal calculi is not increased during pregnancy probably because of the enhanced excretion of inhibitors of stone formation, such as magnesium, citrate, and the glycoprotein nephrocalcin.
Clinical Manifestations
Symptomatic stones during pregnancy are rare, usually presenting in the second or third trimester with acute flank pain radiating to the groin or lower abdomen and hematuria. However, clinical features of renal calculi may be more difficult to interpret in pregnancy because frequent episodes of diffuse, poorly localized abdominal pain and lower urinary tract symptoms are relatively common in normal pregnancy.
From 75% to 85% of stones will pass spontaneously during pregnancy,19 and some women with calculi will have a concomitant UTI. Pregnant women with renal calculi are at greater risk of superimposed pyelonephritis.
The diagnosis of renal calculi in pregnancy is made more difficult because of the accompanying physiologic hydronephrosis and the risks of radiation to the fetus if investigating the urinary tract with computed tomography (CT). Ultrasound will detect hydronephrosis (usually as part of normal pregnancy) and will often detect calculi within the kidney, but ultrasound rarely finds ureteral stones and is less accurate than CT for these purposes. Transvaginal ultrasound should be performed when transabdominal ultrasound is uninformative, to detect distal ureteral stones if pain persists.20 If this remains negative, the woman can be treated symptomatically, with pain relief, hydration, and lying on her side, with the symptomatic side up, enabling relief of pressure on the ureter from the gravid uterus. If symptoms persist and further diagnosis is required, magnetic resonance (MR) urography or low-dose CT (in second or third trimester) can be used, although in our experience, this is rarely needed.
Treatment
Initial management of renal calculi is conservative, with appropriate hydration, antiemetics, and analgesia. Stones pass spontaneously (75% to 85%) in part because of the normally dilated urinary tract in pregnant women. Calcium intake should not be limited in pregnancy. However, women in whom calcium oxalate stones form persistently can limit foods high in oxalate, such as spinach, rhubarb, and chocolate. Urine should always be cultured and appropriate antibiotics administered when a UTI is suspected.
Quantitation of urine calcium, uric acid, or other mineral excretion is not necessary in pregnancy, because specific pharmacologic agents to modify excretion (including thiazides and allopurinol) are contraindicated. Investigations can be completed postpartum.
Urine output should be monitored and renal function assessed. Surgical intervention is considered only when stones cause persistent obstruction, deteriorating renal function, intractable pain or infection, or premature labor unresponsive to other treatment. Cystoscopy with ureteral stenting may be required in pregnancy, and ureteroscopic removal of stones has been reported.21 Lithotripsy is generally contraindicated during pregnancy because of the presumed adverse effect of the shock waves on the fetus; however, some centers have used ureteroscopic laser lithotripsy in pregnant women.22 Extracorporeal shock wave lithotripsy has been reported as an inadvertent procedure in six women in the first month of pregnancy, all of whom subsequently delivered normal infants.23
Postpartum follow-up is important. In the absence of ongoing symptoms, we perform a CT scan of kidney, ureters, and bladder 3 months later. This delay is necessary to eliminate confusion in interpreting the findings; calyceal and ureteral dilation may persist that long after delivery. Women planning a further pregnancy should be assessed for idiopathic hypercalciuria or other causes of renal calculi after a minimum of 3 months postpartum.
Hypertension in Pregnancy
Definitions
There are four major hypertensive disorders related to pregnancy (Fig. 44-1), as follows:
3. Chronic/preexisting hypertension
• Primary
4. Preeclampsia superimposed on chronic/preexisting hypertension
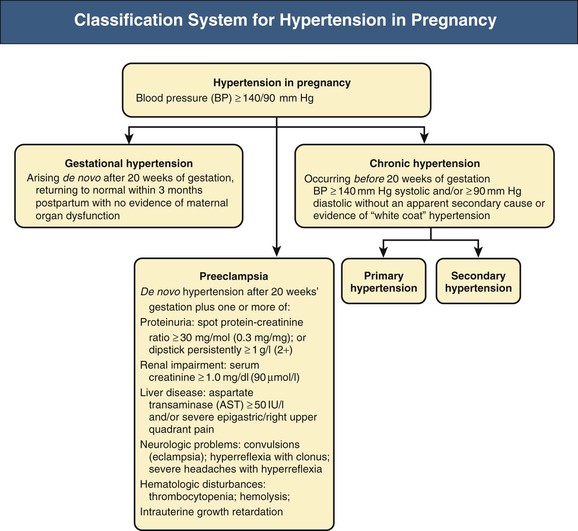
Hypertension in pregnancy is defined as blood pressure of 140/90 mm Hg or higher. Normal pregnancy is characterized by a fall in BP, beginning in the first trimester and reaching a nadir in the second trimester. BP rises toward preconception levels near the end of the third trimester. A BP increment of 30 mm Hg or greater systolic and/or 15 mm Hg diastolic to levels still below 140/90 mm Hg warrants increased frequency of follow-up. By itself, this BP increase does not diagnose gestational hypertension or preeclampsia, but in the presence of proteinuria, this may signify early preeclampsia in some women.
The development of newly elevated BP after 20 weeks’ gestation without evidence of maternal organ dysfunction is known as gestational hypertension. This should usually resolve by 12 weeks postpartum. If hypertension persists beyond 12 weeks postpartum, the diagnosis is likely chronic/preexisting hypertension that has been masked by the physiologic decrease in BP that occurs in early pregnancy. It is important to note, however, that some women with preeclampsia or gestational hypertension may take longer for their high BP to resolve.
Preeclampsia is also hypertension developing in the second half of pregnancy, but this more serious disorder includes accompanying evidence of maternal renal, cerebral, hepatic, or clotting abnormalities and fetal growth restriction (see later discussion).
Chronic/preexisting hypertension is BP of 140/90 mm Hg or higher that antedates pregnancy or is present before the 20th week of pregnancy (on at least two occasions) or persists beyond 12 weeks postpartum. This should ideally be confirmed by 24-hour ambulatory BP monitoring or home self-initiated BP monitoring, to exclude white coat hypertension, which is common in pregnancy.24 Superimposed preeclampsia is the development of proteinuria or new renal dysfunction, thrombocytopenia, neurologic features, or abnormal liver function after 20 weeks’ gestation in a woman with chronic/preexisting hypertension.
The traditional definition of preeclampsia has been hypertension with proteinuria and edema developing after 20 weeks’ gestation. However, edema accompanies two thirds of both normal and preeclamptic pregnancies and is not a particularly useful sign. The detection of proteinuria in the past has also been quite unreliable, and insisting on the finding of proteinuria for this diagnosis ignores the protean manifestations of preeclampsia, as discussed later. In practice, however, the majority of women with multisystem features of preeclampsia also have proteinuria.
Eclampsia (convulsions) is now uncommon in developed countries, with a prevalence of about 0.3% of hypertensive pregnancies. In underdeveloped countries, eclampsia is much more common, with greater risks of maternal mortality and morbidity as well as perinatal mortality.
Epidemiology
Hypertensive disorders of pregnancy are the second most common cause for maternal death worldwide, after hemorrhage.25 Hypertension affects 10% to 12% of all pregnancies,26 and the distribution of causes in a population depends largely on the nature of the obstetric unit assessing that population; tertiary referral units tend to have a higher proportion of severe preeclamptic cases. In general, primary hypertension accounts for about 20%, secondary causes 4%, preeclampsia 35%, and gestational hypertension the remainder of hypertensive disorders in pregnancy. About one in four women with apparent primary hypertension early in pregnancy has white coat hypertension. They present early in pregnancy with apparent chronic hypertension, but their outcomes are better than those with true chronic hypertension. Women with white coat hypertension may be managed without medication through regular home BP monitoring. A small proportion will go on to develop preeclampsia.27
Preeclampsia
Epidemiology
Preeclampsia follows no recognized racial patterns, and no specific genotype-phenotype relation has been associated with this disorder.27 Preeclampsia occurs in 2% to 8% of pregnancies and is a major contributor to maternal mortality worldwide.31 Box 44-2 lists risk factors for preeclampsia. The risk is highest in those with a past history of preeclampsia, with rates ranging from15% to 65% depending on the gestation at onset of the preeclampsia.28,29 Risk of preeclampsia is also high in the first pregnancy, possibly because of limited recent exposure to paternal (sperm) antigens, which may play a role in disease pathogenesis.
An intriguing observation is the reduced risk of preeclampsia in subsequent pregnancies, but a return to the risks of the first pregnancy in women who have a new partner for subsequent pregnancies. This observation, combined with an increased likelihood of preeclampsia in women who have used barrier methods of contraception, raised the possibility of an impaired immunologic response to paternal antigens in such pregnancies. However, this may simply be explained by a longer interpregnancy interval rather than a change of partners, with the incidence increasing after about 7 years between pregnancies.30
Smoking reduces the risk of preeclampsia by a third, but increases the risk of preterm labor, intrauterine growth restriction (IUGR) and placental abruption.31 Obesity increases the likelihood of preeclampsia.
Pathogenesis
The pathogenesis of preeclampsia is complex. The placenta likely causes preeclampsia, with other maternal organs (e.g., kidney) amplifying the disease process (Fig. 44-2). This is supported by the observation that preeclampsia can occur in hydatidiform mole, where the fetus is absent, with the condition resolving when the placenta is removed. The following key mechanisms are involved in the progression to the clinical preeclamptic syndrome:









