Essentials of Diagnosis
General Considerations
It is estimated that the general incidence of primary VUR in the population is 0.4–1.8%. In children who present with a febrile urinary tract infection (UTI), the incidence of VUR increases significantly to 12–50%. Approximately 30–60% of children with VUR will have established renal scars at the time of diagnosis. Moreover, 12% of children with VUR and normal kidneys will develop scarring, irrespective of medical or surgical management. In young children, acquired reflux nephropathy is more common in girls than boys, with a ratio of 4 to 1, similar to the sex prevalence in patients with VUR detected after evaluation for a UTI. In older children, the incidence of reflux nephropathy is equivalent among the sexes, but males appear to be more severely affected. In adults, bilateral nephropathy is more common in men than in women.
With the advent of routine prenatal ultrasonography, many children with VUR are detected prior the development of a UTI. It is estimated that the incidence of VUR in patients with prenatally diagnosed hydronephrosis is approximately 15–20%. In contrast to the sex prevalence of VUR diagnosed following a UTI, VUR detected during evaluation for prenatal hydronephrosis is more common in boys than in girls. It is estimated that 30–50% of these children will have renal abnormalities such as decrease in size, dysmorphic features, or imaging findings similar to renal scarring. The degree of renal abnormalities correlates with the increasing severity of the reflux.
Whether VUR is diagnosed following a UTI or in the evaluation for prenatal hydronephrosis, renal abnormalities/scarring are common at the time of diagnosis. Reflux nephropathy was found to be present in 27% of patients with VUR at the time of presentation. Of children less than 1 year of age, 20% had diffuse abnormalities while 5% had focal abnormalities. In children 1–5 years of age, the same percentage of patients had diffuse abnormalities but 16% had focal abnormalities. Similarly, in children older than 5 years of age, the number of patients with diffuse renal abnormalities was constant at 18% but 20% had focal abnormalities. Dysmorphic kidneys were more commonly seen in children younger than 1 year of age at the time of evaluation, while focal scarring was more commonly seen in older children. In children younger than 1 year of age the male to female ratio with reflux nephropathy was 1.6:1. In contrast, the ratio of male to female changed to 1:4 in older children.
The risk of scarring is greatest in children younger than 1 year of age; reflux nephropathy occurs uncommonly after 5 years of age. It has been observed that new scars developed in 24% of children younger than 2 years of age but in only 10% of children between 2 and 4 years of age and 5% of children older than 5 years of age. These findings suggest the susceptibility of the developing kidney to damage from reflux of infected urine.
Pathogenesis
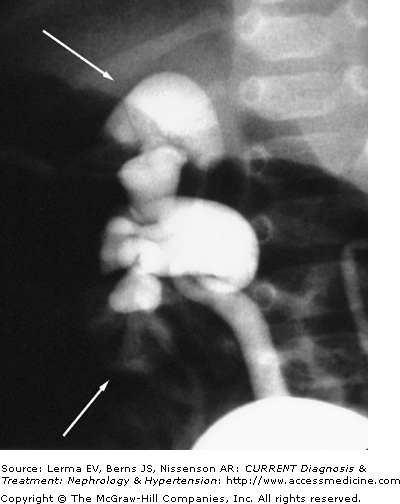
Acquired renal scarring results from an episode or repeated episodes of acute pyelonephritis caused by infected urine in the presence of VUR. Intrarenal reflux allows a retrograde flow of urine into the collecting ducts and into the renal periphery (Figure 39–1). It allows direct transmission of bladder pressures to the renal pelvis, exposing the renal tubules to abnormal pressures. In addition, it exposes the renal parenchyma to urine that is in the bladder, which may have some toxic effects on the kidney cells. More importantly, intrarenal reflux provides a means for urinary pathogens to access renal parenchyma.
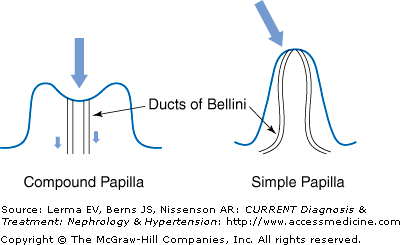
Renal scarring is more likely to occur in the polar regions of the kidney; this is related to the variation in renal papillary morphology. During renal development, different papillary types are formed. Compound papillae arise when two or more individual papillae are joined. This type of papillae is usually found in the renal poles, while simple papillae are more often seen in the other segments of the kidneys. The tips of the papillae project into the renal pelvis and provide a valvular action against the retrograde flow of urine into the collecting tubules. However, the tips of compound papillae are often flattened (Figure 39–2). In animal studies, intrarenal reflux occurs only through the round or gaping ducts of Bellini that open on the flattened (concave) tips of the compound papillae. In contrast, simple papillae tend to have convex surfaces with a slit-like ductal opening and, thus, do not permit reflux. These papillary configurations and their predisposition for intrarenal reflux have also been observed in children with VUR. Renal scarring occurs only in area with intrarenal reflux. Moreover, physical distortion such as from hydronephrosis, high-pressure voiding, or adjacent renal scarring could convert nonrefluxing to refluxing papillae.
Renal scarring appears to occur more often when both VUR and infection are present. In a pig model, it has been observed that renal scarring does not occur when VUR is created on one side. However, when a bacterial infection is introduced into the bladder, renal scarring develops only on the side with VUR, while the nonrefluxing renal unit remains normal. This process begins with small discrete abscesses developing in individual tubules, which later coalesce into a confluent mass. Acutely, the interstitium is infiltrated with inflammatory cells, inducing tubular destruction and a variable amount of interstitial fibrosis. Within 2 weeks, the active interstitial inflammatory cell infiltration becomes patchier in distribution, with lymphoid follicles becoming more prominent and irreversible scarring being established. By 3 weeks, scar contraction occurs. These histopathologic findings are identical to those found in children with reflux nephropathy. Furthermore, treatment with antibiotics during the first week after infection appears to limit inflammation, and consequently, scar formation. Only fine linear scars extending through the cortex and small dimpling of the renal surface are evident if appropriate antibiotic therapy is instituted during the early inflammatory phase.
Bacterial virulence also plays an essential role in the development of renal scarring. Bacteria express factors that facilitate adhesion to the uroepithelium and increase the propensity for renal injury. Pyelonephritogenic strains of Escherichia coli commonly have fimbriae that fail to react with mannose on the host cell surface and express P blood group ligands on their fimbriae. Other virulence factors such as hemolysin and K-antigen augment the host inflammatory response and the degree of tissue destruction. Bacteria that express several virulence factors can cause acute pyelonephritis in the absence of VUR. However, in the presence of VUR, less virulent bacteria cause similar episodes of pyelonephritis. It is presumed that VUR compromises host defense mechanisms such as the antegrade flow of urine from the kidneys and the normal configuration of the papillae, which then allow the bacteria to have direct assess into the renal tubules.
Infection activates a cascade of mediators, which leads to renal epithelial cells damage. In animal models, bacterial infection that reaches the kidney rapidly induces the activation of complement, granulocytic aggregation, and capillary obstruction. Phagocytosis of the bacterial endotoxins causes the release of proteolytic enzymes from granulocytes, inducing cell injury and death. More importantly, ischemia and reperfusion injury induce further renal damage through the release of superoxide and other free-oxygen radicals, which in turn destroy the lipid cell membranes. The ischemia results from a focal decrease in renal blood flow, due in part to a transient elevation in renal vein renin during the early inflammatory phase of pyelonephritis, compression of the vasa recta and peritubular capillaries by edema, and obstruction of the renal microvasculature by platelets and granulocyte aggregations. Free-oxygen radicals are also produced by the respiratory burst from neutrophilic leukocytes engaging in bacterial phagocytosis and by the metabolism of products produced by the anaerobic metabolism of adenosine triphosphate. The effects of ischemia and reperfusion injury on the renal epithelial cells could be limited by modulating the formation of free-oxygen radicals through the administration of exogenous superoxide dismutase, complement depletion, or pretreatment with allopurinol. Currently, the efficacy of these methods in modulating renal injury induced by pyelonephritis in humans remains unknown.
Renal scarring can also occur in the absence of infection. It is believed that the reflux sterile urine can induce an inflammatory response in the renal parenchyma that leads to scarring. From radiographic observations, it is noted that VUR not only goes into the collecting ducts (ie, pyelotubular) but also extends into the interstitium (ie, pyelointerstitial, either via the collecting ducts themselves or through a direct break into the epithelial lining of the collecting system. It is known that when it escapes outside the epithelium-lined surface, sterile urine can produce a severe inflammatory reaction, leading to a fibrotic response and subsequent scarring. This process of renal damage might be slower compared to that induced by the backflow of infected urine, but nevertheless, can cause the same extensive damage. Histologic evidence concerning the effects of sterile refluxed urine on the renal parenchyma has been seen from pathologic specimens from children and adults with severe renal scarring and VUR without any documented urinary tract infection.