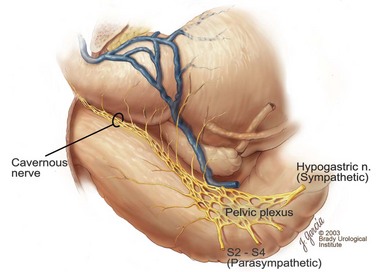
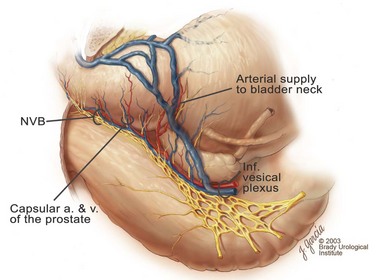
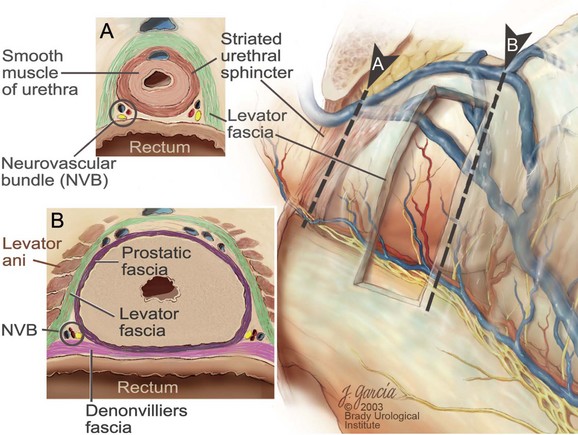
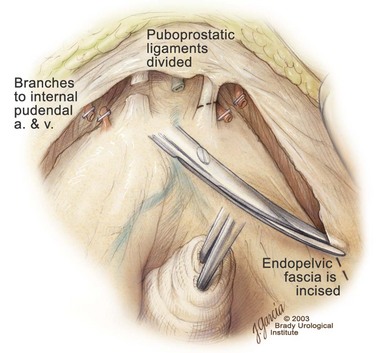
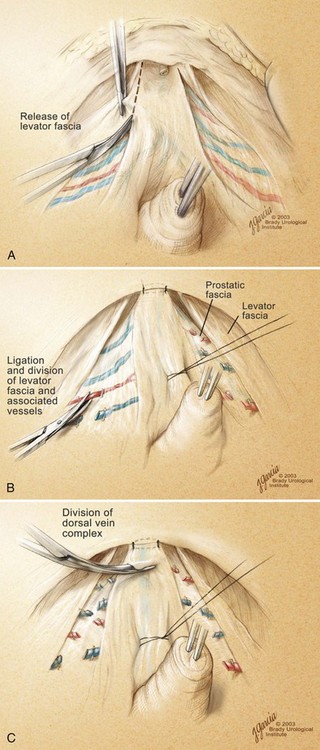
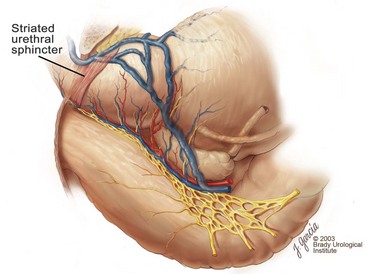
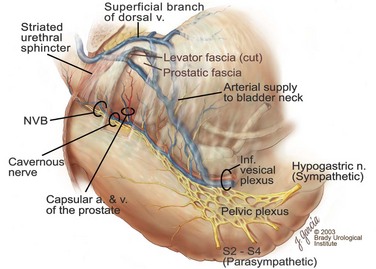
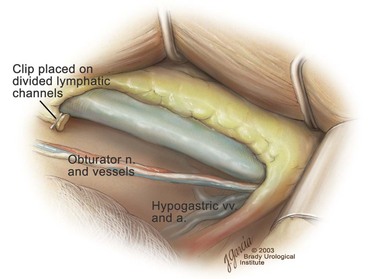
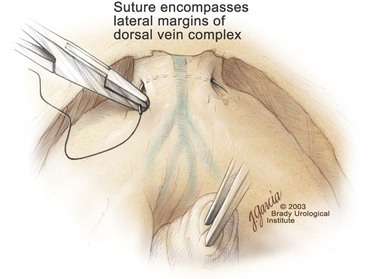
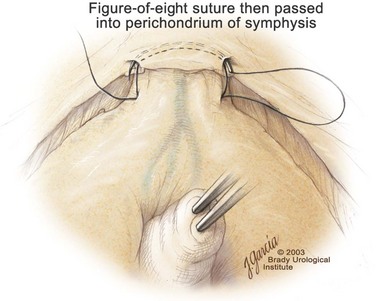
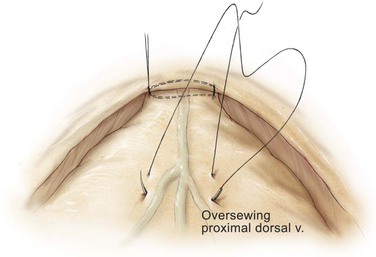
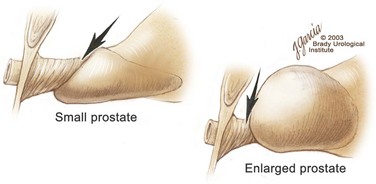
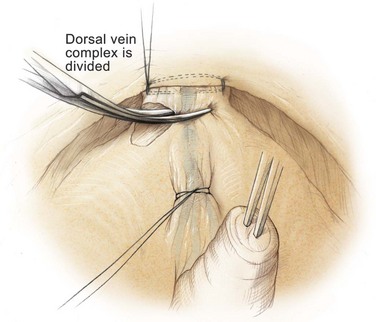
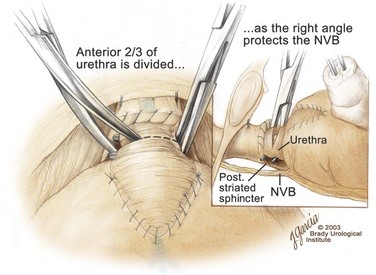
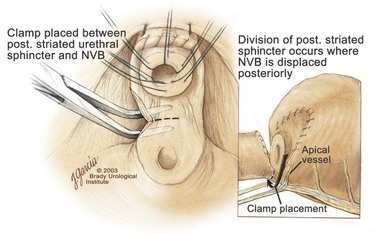
Edward M. Schaeffer, MD, PhD, Alan W. Partin, MD, PhD, Herbert Lepor, MD, Patrick C. Walsh, MD There is no better way to cure cancer that is confined to the prostate than total surgical removal. Radical prostatectomy is the only form of treatment for localized prostate cancer that has been shown in a randomized controlled trial to reduce progression to metastases and death from the disease (Holmberg et al, 2002; Bill-Axelson et al, 2008). Furthermore, on the basis of improved understanding of the periprostatic anatomy, today there is less bleeding and improved rates of postoperative continence and potency (Walsh, 1998, 2000; Nielsen et al, 2008). Technically, radical retropubic prostatectomy is one of the most difficult operations in the field of urology. The three goals of surgery, in order of importance, are cancer control, preservation of urinary control, and preservation of sexual function. Great skill and experience in the selection of surgical candidates and operative technique are necessary to achieve all three. This chapter summarizes the authors’ experience with the hope that it will shorten the reader’s learning curve. A video demonstrating a detailed description of the surgical technique is also available (Walsh and Garcia, 2004). The veins of the prostate drain into the Santorini plexus. It is necessary to have a complete understanding of these veins to avoid excessive bleeding and to ensure a bloodless field in exposing the membranous urethra and the apex of the prostate. The deep dorsal vein leaves the penis under the Buck fascia between the corpora cavernosa and penetrates the urogenital diaphragm, dividing into three major branches: the superficial branch and the right and left lateral venous plexuses (Reiner and Walsh, 1979) (Fig. 102–1). The superficial branch, which travels between the puboprostatic ligaments, is the centrally located vein overlying the bladder neck and prostate. This vein is easily visualized early in retropubic operations and has communicating branches over the bladder itself and into the endopelvic fascia. The superficial branch lies outside the anterior prostatic fascia. The prostate receives arterial blood supply from the inferior vesical artery. According to Flocks (1937), after the inferior vesical artery provides small branches to the seminal vesicle and the base of the bladder and prostate, the artery terminates in two large groups of prostatic vessels: the urethral and capsular groups (Fig. 102–2). The urethral vessels enter the prostate at the posterolateral vesicoprostatic junction and supply the vesical neck and periurethral portion of the gland. The capsular branches run along the pelvic sidewall in the lateral pelvic fascia posterolateral to the prostate, providing branches that course ventrally and dorsally to supply the outer portion of the prostate. The capsular vessels terminate as a small cluster of vessels that supply the pelvic floor. On histologic examination, the capsular arteries and veins are surrounded by an extensive network of nerves (Walsh and Donker, 1982; Walsh et al, 1983; Lue et al, 1984; Lepor et al, 1985). These capsular vessels provide the macroscopic landmark to aid in the identification of the microscopic branches of the pelvic plexus that innervate the corpora cavernosa. The major arterial supply to the corpora cavernosa is derived from the internal pudendal artery. However, pudendal arteries can arise from the obturator, inferior vesical, and superior vesical arteries. Because these aberrant branches travel along the lower part of the bladder and anterolateral surface of the prostate, they are divided during radical prostatectomy. This may compromise arterial supply to the penis, especially in older patients with borderline penile blood flow (Breza et al, 1989; Polascik and Walsh, 1995; Rogers et al, 2004). The autonomic innervation of the pelvic organs and external genitalia arises from the pelvic plexus, which is formed by parasympathetic, visceral, efferent, preganglionic fibers that arise from the sacral center (S2 to S4) and sympathetic fibers via the hypogastric nerve from the thoracolumbar center (Walsh and Donker, 1982; Lue et al, 1984; Lepor et al, 1985; Schlegel and Walsh, 1987; Walsh, 2007) (see Fig. 102–1). The pelvic plexus in men is located retroperitoneally beside the rectum 5 to 11 cm from the anal verge and forms a fenestrated rectangular plate that is in the sagittal plane with its midpoint at the level of the tip of the seminal vesicle. The branches to the membranous urethra and corpora cavernosa also travel outside the prostatic capsule in the lateral pelvic fascia dorsolaterally between the prostate and rectum (see Fig. 102–1). Although these nerves are microscopic, their anatomic location can be estimated intraoperatively by use of the capsular vessels as a landmark. This structure, which is referred to here as the neurovascular bundle, has been termed the neurovascular bundle of Walsh (Stedman’s Medical Dictionary, 2000) (see Fig. 102–2). As emphasized by Takenaka and colleagues (2004) and Costello and colleagues (2004), the cavernous branches join the capsular arteries and veins in a spraylike distribution to form the neurovascular bundle 20 to 30 mm distal to the junction of the bladder and prostate (see Fig. 102–2). The neurovascular bundles are located in the lateral pelvic fascia between the prostatic fascia and the levator fascia. At the apex of the prostate, the branches of the nerves to the cavernous bodies and striated sphincter also have a spraylike distribution both anteriorly and posteriorly with wide variation (Costello et al, 2004; Takenaka et al, 2005a). After piercing the urogenital diaphragm, the nerve branches pass behind the dorsal penile artery and dorsal penile nerve before entering the corpora cavernosa (Walsh and Donker, 1982). The external sphincter, at the level of the membranous urethra, is often depicted as a “sandwich” of muscles in the horizontal plane. However, Oelrich (1980) demonstrated clearly that the striated urethral sphincter with its surrounding fascia is a vertically oriented tubular sheath that surrounds the membranous urethra. In utero, this sphincter extends without interruption from the bladder to the perineal membrane. As the prostate develops from the urethra, it invades and thins the sphincter muscle, causing a reduction or atrophy of some of the muscle (Fig. 102–3). (© Brady Urological Institute.) In the adult the fibers at the apex of the prostate are horseshoe shaped and form a tubular, striated sphincter surrounding the membranous urethra. Near the apex of the prostate, the edges fuse in the midline posteriorly (Fig. 102–4A). Thus as Myers (1987) has shown, the prostate does not rest on a flat, transverse urogenital diaphragm like an apple on a shelf, with no striated muscle proximal to the apex. Rather, the external striated sphincter is more tubular and has broad attachments over the fascia of the prostate near the apex. This has important implications in the apical dissection and reconstruction of the urethra for preservation of urinary control postoperatively (Walsh et al, 1990). The striated sphincter contains fatigue-resistant, slow-twitch fibers that are responsible for passive urinary control. Active continence is achieved by voluntary contraction of the levator ani musculature, which surrounds the apex of the prostate and membranous urethra. Some fibers of the levator ani (levator urethrae, pubourethralis) surround the proximal urethra and the apex of the prostate and insert into the perineal body in the midline posteriorly (Myers, 1991, 1994). The pudendal nerve provides the major nerve supply to the striated sphincter and levator ani. When patients are instructed to perform sphincter exercises postoperatively, they are actually contracting the levator ani musculature. However, because the striated urethral sphincter has similar innervation, patients are exercising this important muscle as well. Somatic motor nerves traveling through the pelvic plexus provide additional innervation to the pelvic floor musculature (Zvara et al, 1994; Costello et al, 2004, Takenaka et al, 2005a). The prostate is covered with three distinct and separate fascial layers: Denonvilliers fascia, the prostatic fascia (also called the capsule of the prostate), and the levator fascia. Denonvilliers fascia is a filmy, delicate layer of connective tissue located between the anterior walls of the rectum and prostate (Fig. 102–4B). This fascial layer extends cranially to cover the posterior surface of the seminal vesicles and lies snugly against the posterior prostatic capsule. This fascia is most prominent and dense near the base of the prostate and the seminal vesicles and thins dramatically as it extends caudally to its termination at the striated urethral sphincter. On microscopic examination, it is impossible to discern “posterior” and “anterior” layers of this fascia (Jewett et al, 1972). For this reason, one must excise this fascia completely to obtain an adequate surgical margin. In addition to Denonvilliers fascia, the prostate is also invested with the prostatic fascia and levator fascia. Anteriorly and anterolaterally, the prostatic fascia is in direct continuity with the parenchyma of the prostate. The major tributaries of the dorsal vein of the penis and Santorini plexus travel within the anterior prostatic fascia. Laterally, the prostatic fascia fuses with the levator fascia, which covers the pelvic musculature, to form the lateral pelvic fascia (Fig. 102–5) (Myers, 1991, 1994). Posterolaterally, the levator fascia separates from the prostate to travel immediately adjacent to the pelvic musculature surrounding the rectum. The prostate receives its blood supply and autonomic innervation between the layers of the levator fascia and prostatic fascia (see Figs. 102-4B and 102-5). (© Brady Urological Institute.) In an effort to avoid injury to the dorsal vein of the penis and Santorini plexus during radical perineal prostatectomy, the lateral and anterior pelvic fasciae are reflected off the prostate. This accounts for the reduced blood loss associated with radical perineal prostatectomy. In performing radical retropubic prostatectomy, the prostate is approached from outside these fascial investments. For this reason, the dorsal vein complex must be ligated and the lateral pelvic fascia must be divided (Walsh et al, 1983). The observation that up to 15% to 20% of men develop an inguinal hernia following radical retropubic prostatectomy implied that this surgical procedure directly predisposes to the development of hernias (Regan, 1996; Lodding, 2001; Nielsen and Walsh, 2005). Approximately 15% of men undergoing radical prostatectomy will have a coexisting inguinal hernia detected if an appropriate inguinal examination is performed (Lepor and Robbins, 2007). In most of these cases, the inguinal hernias are asymptomatic. These observations suggest that a radical retropubic prostatectomy may transform an asymptomatic inguinal hernia into a symptomatic hernia. Therefore examination of the inguinal canal with Valsalva should be performed, enabling properitoneal hernia repairs at the time of the radical prostatectomy. During this delay, patients may be offered the opportunity to donate autologous blood, although the utilization of autologous transfusions at our institutions has declined as continuous modifications to our technique have reduced blood loss. One of the limitations of autologous blood donation is that many donated units are not transfused if the hematocrit is higher than 30% (Goad et al, 1995). Therefore hemodilution and erythrocyte stimulating proteins have been recommended as alternative blood management strategies (Gilbert and Smith, 2000). Erythrocyte-stimulating proteins, which are not approved by the U.S. Food and Drug Administration, have been shown to raise the hematocrit on average percentage points, diminishing postprostatectomy anemia (Rosenblum et al, 2000). The relatively higher discharge hematocrit attributable to the use of erythrocyte stimulating proteins affects the pace of recovery because postoperative hematocrit has been shown to influence both time to work and return to activities (Sultan et al, 2006). The American Urological Association recently published best practice guidelines for the use of prophylactic antibiotics for urologic surgical procedures (Wolf et al, 2008). The recommended prophylactic regimens for open or laparoscopic surgery involving entry into the urinary tract are a first- or second-generation cephalosporin or an aminoglycoside in combination with metronidazole or clindamycin. The duration of therapy should be no longer than 24 hours. The authors routinely use a first-generation cephalosporin alone and have an infection rate much less than 1%. While donating blood and immediately before surgery, patients should avoid taking high doses of vitamin E, aspirin, nonsteroidal anti-inflammatory agents, omega-3 fish oils, and thienopyridines (Plavix and Ticlid), which interfere with platelet function. Products that are not medically indicated should be discontinued at least 1 week before surgery. The preoperative management of anticoagulants and antiplatelet agents that are used for the treatment of specific medical conditions such as prosthetic valves, atrial fibrillation, and coronary artery stent implants should be performed in conjunction with the internist or cardiologist. With nearly 1 million coronary interventions occurring every year and the majority including an implant of a drug-eluting stent, managing prescribed antiplatelet therapy can create a dilemma balancing the risk of stent stenosis and perioperative bleeding. In the majority of cases, stent thrombosis is a catastrophic event resulting in life-threatening complications (Cutlip et al, 2001). A reasonable compromise for men with drug-eluting stents undergoing a radical retropubic prostatectomy is to preoperatively discontinue the thienopyridine while continuing aspirin therapy and restarting the thienopyridine as soon as clinically indicated (Grines et al, 2007). A spinal or epidural anesthetic is preferable for this procedure. Regional anesthesia is associated with less blood loss and a lower frequency of pulmonary emboli (Peters and Walsh, 1985; Shir et al, 1995). The anesthesiologist is encouraged to maintain relative hypotension with systolic blood pressure of no more than 100 mm Hg and to limit the replacement of crystalloid to 1500 mL until the prostate is removed (Davies et al, 2004). The patient is placed in the supine position with the table flexed to increase the distance between the umbilicus and pubis. The pelvic lymph node dissection is performed before the radical prostatectomy. The dissection is initiated on the ipsilateral side of the major tumor in the prostate by dividing the adventitia over the external iliac vein (Fig. 102–6). The lymphatics overlying the external iliac artery are preserved. The dissection proceeds beneath the external iliac vein out to the pelvic sidewall and then inferiorly to the femoral canal, where the lymphatic channels are ligated at a convenient point. There is no need to remove the Cloquet node. The dissection then proceeds superiorly along the pelvic sidewall to the bifurcation of the common iliac artery, where the lymph nodes in the angle between the external iliac and hypogastric arteries are removed. Next, the obturator lymph nodes are removed with care to avoid injury to the obturator nerve. The obturator artery and vein are skeletonized but are usually left undisturbed and are not ligated unless excessive bleeding occurs. The dissection then continues down to the pelvic floor, exposing the hypogastric veins. This extended dissection removes more lymph nodes than in a more limited dissection, improving staging and providing potential therapeutic benefit in some patients (Allaf et al, 2004; Palapattu et al, 2004). A similar procedure is performed on the opposite side. If the patient has a well-differentiated to moderately well–differentiated tumor (Gleason grade < 8) and the lymph nodes are normal to palpation, frozen-section analysis is not performed (Sgrignoli et al, 1994; Cadeddu et al, 1997). (© Brady Urological Institute.) The endopelvic fascia is entered where it reflects over the pelvic sidewall, well away from its attachments to the bladder and prostate (Fig. 102–7). The point of incision is where the fascia is transparent, revealing the underlying levator ani musculature. After the fascia has been opened, one can usually visualize the bulging lateral venous plexus of Santorini, which is located medially. Therefore an incision in the endopelvic fascia too close to the bladder or the prostate risks laceration of these veins, with potential severe blood loss. Beneath this venous complex lie the prostatic arteries and the branches of the pelvic plexus that course toward the prostate, urethra, and corpora cavernosa. The fibrofatty tissue covering the superficial branch of the dorsal vein and puboprostatic ligaments is gently teased away to prepare for division of the ligaments without injury to the superficial branch of the dorsal vein. After the superficial branch has been dissected away from the medial edge of the ligaments, it is coagulated and divided. After all fibrofatty tissue has been removed, a sponge stick is used to displace the prostate posteriorly, and scissors are used to divide each ligament (see Fig. 102–7). The dissection should continue down far enough to expose the juncture between the apex of the prostate and the anterior surface of the dorsal vein complex at the point where it will be divided. The pubourethral component of the complex must remain intact to preserve the anterior fixation of the striated urethral sphincter to the pubis (Burnett and Mostwin, 1998). Arterial insufficiency is a factor contributing to erectile dysfunction in patients after nerve-sparing radical retropubic prostatectomy. One source for this insufficiency may arise from accessory pudendal arteries that travel over the anterolateral surface of the prostate. These arteries have been found in 70% of cadaveric dissections and in 7% of patients by selective internal pudendal angiography. In the authors’ experience, large visible accessory pudendal arteries are present in 4% of men (Rogers et al, 2004). Because release of accessory arteries may be associated with significant blood loss necessitating prompt ligation and division of the dorsal vein complex, it is useful to have the contralateral dissection completed before accessory vessels are released. For this reason, when these vessels appear to be prominent and are present unilaterally, the endopelvic fascia and puboprostatic ligament on the contralateral side should be divided first. The surgical technique for preservation of the arteries begins with division of the endopelvic fascia lateral to the vessels and division of the puboprostatic ligament (the vessels are beneath the puboprostatic ligament) (Rogers et al, 2004). Next, a vessel loop is used to elevate the artery and then with sharp dissection and a right-angled clamp, the accessory artery is released from its investing fascia (Fig. 102–8). As the dissection proceeds, venous tributaries are divided. At times, there may be substantial blood loss from the venous complex over the prostate; hemostasis, however, is usually easily obtained with a 2-0 running absorbable suture. The dissection must extend caudally beyond the site at which the dorsal vein complex will be divided. The goal is to divide the complex with minimal blood loss while avoiding damage to the striated sphincter and inadvertent entry into the anterior apex of the prostate. With use of the sponge stick to push the prostate posteriorly, a 3-0 Monocryl suture is passed superficially through the dorsal vein complex just distal to the apex of the prostate (Fig. 102–9). In placing this stitch, the surgeon should face the head of the table, holding the needle driver against the pubis perpendicular to the patient. Next, the needle is reversed in the needle holder and the same suture is placed through the perichondrium of the pubic symphysis (Fig. 102–10). Once this horizontal mattress suture is tied, three important goals are accomplished: (1) control of much of the venous bleeding without a “bunching” effect—this flat surface is much easier to divide; (2) recapitulation of the puboprostatic ligaments to provide additional anterior support of the striated sphincter; and (3) fixation of the dorsal vein complex anteriorly. This enables the surgeon to visualize the plane on the anterior apex of the prostate during division of the dorsal vein complex. The suture is not cut; it will be used once the dorsal vein is divided to oversew bleeders. A figure-of-eight 2-0 chromic suture is next placed on the anterior surface of the prostate near the bladder neck to reduce bleeding from the proximal dorsal veins, which can be excessive in some patients who have incompetent venous valves (Fig. 102–11). (© Brady Urological Institute.) (© Brady Urological Institute.) (© Brady Urological Institute.) With recognition that high release of the levator fascia at the apex can result in earlier recovery of potency in selected patients (Nielsen et al, 2008), an alternative technique using high anterior release of the neurovascular bundle, before division of the dorsal vein, is also described. As Myers (1991) has pointed out, there is marked variability in the shape of the apex of the prostate (Fig. 102–12). For this reason, it is not wise to pass an instrument bluntly through the complex blindly. Rather, the dissection should be approached by direct division and visual assessment of the landmarks. With the application of downward pressure on the anterior surface of the prostate with a sponge stick, Metzenbaum scissors are used to divide the complex. This is usually started on the left edge of the complex where the junction with the apex of the prostate can usually be seen well (Fig. 102–13). Because the distal complex is fixed anteriorly, with downward pressure on the sponge stick, the exact plane between the juncture of the anterior surface of the prostate and the striated musculature can be visualized. Here the 4.5-power loupes enable the surgeon to distinguish tissue textures and avoid positive margins. This is the most common site for positive surgical margins because it can be difficult to identify the anterior apical surface of the prostate. It also helps to rest the scissors on the anterior apex to find the correct plane. Figure 102–12 As Myers (1991) has pointed out, there is marked variability in the shape of the apex of the prostate. In patients with a small prostate it can have a gentle slope, whereas in patients with an enlarged prostate, there can be an abrupt 90-degree angle. Knowledge of this variability is important to avoid excising excessive amounts of the striated sphincter. (© Brady Urological Institute.) Figure 102–13 With a sponge stick depressing the prostate posteriorly, the dorsal vein complex is divided beginning at the left edge. (© Brady Urological Institute.) There is one important caveat in performing this maneuver. As the dissection proceeds posteriorly, if the striated sphincter is divided too close to the apex of the prostate, there is risk that the neurovascular bundle may be damaged. As the neurovascular bundle approaches the apex of the prostate, it is often fixed medially beneath the striated sphincter by an apical vessel (Walsh et al, 2000b). For this reason, the lateral edges of the sphincter along the urethra should be divided obliquely midway between the apex of the prostate and the pelvic floor. Absolute control of venous bleeding from the dorsal vein complex is mandatory so that the remainder of the procedure can be performed in a bloodless field. To achieve hemostasis, the 3-0 Monocryl suture on a (© Brady Urological Institute.) Finally, to control back-bleeding from the anterior surface of the prostate, the edges of the proximal dorsal vein complex on the anterior surface of the prostate are sewn in the shape of a V with a running 2-0 absorbable suture (Fig. 102–15). If one tries to pull these edges together in the midline, the neurovascular bundles can be advanced too far anteriorly on the prostate (Walsh et al, 2000b). If one begins by placing a figure-of-eight suture at the bladder neck before dividing the dorsal vein complex and then uses the same suture to oversew the proximal dorsal vein, there is less back-bleeding. (© Brady Urological Institute.) By gently displacing the prostate posteriorly with a sponge stick, the prostatourethral junction should be well visualized. The lateral bands of the striated musculature should be released as described earlier at their midpoint to free up as much of the urethra as possible. A right-angled clamp is then passed around the smooth musculature of the urethra near the apex of the prostate to ensure that the urethra is transected as close to the apex as possible (Fig. 102–16). With scissors, the anterior two thirds of the urethra is divided with care to avoid damage to the Foley catheter. This provides excellent exposure for placement of five sutures in the distal urethral segment at the 12-, 2-, 5-, 7-, and 10-o’clock positions. With 3-0 Monocryl on a Figure 102–16 A right-angled clamp is placed around the smooth muscle of the urethra close to the apex of the prostate. Note in the inset that the neurovascular bundles (NVBs) are protected from injury by the posterior component of the striated sphincter, which is still intact. Also see Figure 102–5. (© Brady Urological Institute.) (© Brady Urological Institute.) After the sutures are placed, the catheter is removed. The 6-o’clock suture is placed through the mucosa of the urethra, from the outside to the inside. The posterior band of urethra is now divided to expose the posterior portion of the striated urethral sphincter complex (see Fig. 102–17). The posterior wall of the striated sphincter complex is composed of skeletal muscle and fibrous tissue. Identification and precise division of this complex are important in (1) obtaining adequate margins of resection for apical lesions, (2) identifying the correct plane on the anterior wall of the rectum to ensure that all layers of Denonvilliers fascia are excised, (3) avoiding blunt trauma to the neurovascular bundles that are located immediately posteriorly, and (4) preserving urinary continence. To divide the posterior portion of the sphincter safely, a right-angled clamp is passed immediately beneath the left edge of this complex (Fig. 102–18). The clamp should pass midway between the apex of the prostate and the urethra. If it is passed too close to the apex of the prostate, it may damage the neurovascular bundle. However, midway, the neurovascular bundle is located more posteriorly and is beneath the right-angled clamp (Walsh et al, 2000b) (see Fig. 102–5). (© Brady Urological Institute.) The purpose of this approach is to speed up the recovery of sexual function and continence by reducing traction on the branches of the nerves to the cavernous bodies and striated sphincter or to avoid inadvertent transection of the small branches that travel anteriorly (Costello et al, 2004; Takenaka et al, 2004, 2005a; Horninger et al, 2005; Menon et al, 2005; Montorsi et al, 2005). However, because there is less soft tissue at the apex, the risk of positive surgical margins could be increased by this approach. The authors select candidates for this approach using parameters that identify patients with a low risk of extraprostatic extension in the region of the neurovascular bundle (Tsuzuki et al, 2005), and in the authors’ hands, they have not noted an increased rate of positive surgical margins (for a detailed discussion of Tsuzuki parameters, see later). The procedure begins after the endopelvic fascia has been opened as previously described. The levator fascia over the anterior apex of the prostate is incised as suggested by Takenaka (2005b), and the incision is extended distally along the lateral edge of the dorsal vein complex on the anterolateral surface of the prostate, preserving the underlying prostatic fascia (Fig. 102–19A). The prostatic fascia is the glistening white fascia immediately below the veins that travel along the lateral surface of the prostate. To identify the correct plane and to avoid inadvertent incision into the prostate, it is essential to have excellent visualization and magnification (4.5-power loupes). In many patients, these venous tributaries must be divided and controlled. Cautery should not be used. Instead, hemostasis should be achieved by use of small hemoclips. As the dissection proceeds distally, the levator fascia should be released from the lateral shoulders of the prostate, with care taken not to enter the underlying prostatic fascia. Because the recovery of sexual function at 12 months following unilateral high release was the same as with bilateral high release, the authors now routinely perform it on only one side, the side with the most favorable pathology. Once the dissection has extended distally beyond the apex of the prostate, the dorsal vein is ligated distally and proximally as described previously (Fig. 102–19B). Next, while the prostate is immobilized by the dorsal vein complex, the prostate is turned on the side using a sponge stick and the levator fascia and associated veins are released further from the prostatic fascia posterolaterally, exposing and releasing the neurovascular bundle. Next the dorsal vein complex, which has been skeletonized by these maneuvers, is ligated further with the Monocryl suture to reduce blood loss when it is divided. The prostate is then displaced posteriorly by the sponge stick, and the dorsal vein complex is divided between the two ties down to the urethra (Fig. 102–19C). Because the levator fascia has previously been released, prominent neurovascular bundles can usually be visualized lateral to the urethra. Bleeding from the distal dorsal vein complex is controlled with the 3-0 Monocryl suture. Residual back-bleeding from the proximal dorsal vein on the anterior surface of the prostate is controlled with the 2-0 chromic suture. Because less of the striated sphincter is excised by this technique, the bleeding edges can usually be brought together in the midline. Next, the urethra is transected and the urethral sutures placed. The posterior component of the striated sphincter is transected. If high release of the levator fascia was only performed unilaterally, insert a right-angled clamp under the levator fascia at the apex on the side contralateral to the high release. This facilitates identification of the same dissection plane beneath the levator fascia as the contralateral side without the need for approaching the plane by first dividing the levator fascia from the outside (Fig. 102–20). At this point, as described by Masterson and colleagues (2008), the neurovascular bundle is released from the lateral surface of the prostate to just above the seminal vesicles. This approach avoids traction on the neurovascular bundles and makes the subsequent steps in preservation of the neurovascular bundles much easier (Fig. 102–21). Today, in most men who are candidates for surgery, it is safe to preserve both neurovascular bundles and rarely necessary to excise both of them (Walsh, 2001). With improved surgical techniques and the availability of phosphodiesterase type 5 inhibitors, most healthy potent men younger than 65 years should be potent after surgery. As discussed earlier, the neurovascular bundle is outside the prostate between layers of lateral pelvic fascia (the levator fascia and prostatic fascia). If nerve sparing is performed correctly, the prostatic fascia must remain on the prostate. This is called an interfascial dissection. Furthermore, when cancer extends through the capsule, it rarely penetrates more than 1 to 2 mm, and this much tissue is often present even when the neurovascular bundle is preserved (Hernandez et al, 2005). As Costello and colleagues (2004) have shown, the cavernous branches are positioned posterior to the capsular vessels and the nerves immediately adjacent to the prostatic capsule are nerves going into the prostate, not the cavernous nerves. To avoid bleeding from the capsular arteries and veins, some surgeons dissect beneath the prostatic fascia. This is called an intrafascial dissection. Because this plane is directly on the prostatic parenchyma, the risk of positive surgical margins is high. For this reason, this approach should never be used. Many well-meaning surgeons believe that if they excise the neurovascular bundle, they can avoid a positive surgical margin. Unfortunately, this is not the case because the neurovascular bundle is not the most common site for positive margins. Rather, the most common site is the apex, followed by posterior and then posterolateral. In 22% the positive margins are multiple. In multiple studies, it has been demonstrated that the rate of positive margins in nerve-sparing and non–nerve-sparing procedures is the same (Ward et al, 2004). In Walsh’s hands, the neurovascular bundles are preserved bilaterally in 87% of patients; one neurovascular bundle is excised in 13%. This has resulted in an overall rate of positive surgical margins of approximately 5% (Epstein, 2001). The neurovascular bundles are rarely if ever excised bilaterally. The authors have always believed that if there is capsular penetration bilaterally to the point where it is necessary to excise both neurovascular bundles, the patient most likely has distant dissemination and is not curable. Between 1986 and 1999, only seven potent men had both neurovascular bundles excised. Four were not cured because they had positive lymph nodes, positive seminal vesicles, or positive surgical margins elsewhere. In the three other patients, it was not necessary to excise both neurovascular bundles because there was no capsular penetration on one side. How does one decide when and where to excise the neurovascular bundle? Preoperatively, no definite decision is made. Consideration is given to the status of sexual function, but in impotent patients, the authors do not always excise the neurovascular bundles because there is evidence that the bundles provide both somatic and autonomic innervation to the continence mechanism and that patients who undergo excision of both neurovascular bundles have more incontinence than do patients in whom the neurovascular bundles are preserved (Nelson et al, 2003). Consideration is also given to other important factors such as the presence of a palpable apical lesion and a high probability of capsular penetration based on the Partin tables (Partin et al, 1997, 2001). However, no final decision is made until the time of surgery. When the endopelvic fascia is opened, if induration is palpable in the lateral pelvic fascia, the neurovascular bundle on that side is widely excised. If there is no induration but the neurovascular bundle appears to be fixed to the prostate at the time it is being released, it is also excised. However, the final decision about preservation or wide excision of the neurovascular bundle does not need to be made until the prostate is removed. If there appears to be inadequate tissue over the posterolateral surface after the prostate has been removed, the neurovascular bundle can then be widely excised. For surgeons who are not comfortable or experienced in using intraoperative findings, Tsuzuki and colleagues (2005) have defined preoperative parameters to identify patients with a higher likelihood of extraprostatic extension in the region of the neurovascular bundle. If patients have two or more of the following criteria, the risk of extraprostatic extension in the region of the neurovascular bundle on that side is greater than 10%: prostate-specific antigen (PSA) level above 10 ng/mL, Gleason score higher than 6, average percentage of biopsy core on the side involved greater than 20%, percentage of cores with tumor on that side greater than 33%, or an abnormal finding on digital rectal examination. The authors use these parameters when selecting candidates for high anterior release of the neurovascular bundles.
Radical Retropubic Prostatectomy: Surgical Anatomy
Venous and Arterial Anatomy
Pelvic Plexus
Striated Urethral Sphincter
Pelvic Fascia
Surgical Technique
Preoperative Preparation
Anesthesia, Incision, and Lymphadenectomy
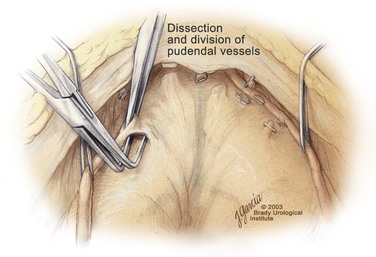
Incision in Endopelvic Fascia
Division of the Puboprostatic Ligaments
Preservation of Accessory Pudendal Arteries
Ligation of the Dorsal Vein Complex
Apical Dissection
Standard Division of the Dorsal Vein Complex
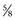 -circle needle that was placed previously is used to oversew the superficial edges of the striated urethral sphincter–dorsal vein complex (Fig. 102–14). In placing this running suture, the surgeon should face the head of the table, holding the needle driver against the pubis perpendicular to the patient. If the needle driver is held loosely, the superficial edges of the complex can be easily approximated, forming a hood over the anterior urethra. Circumflex veins are often present at the posterior edge of the complex at the 5- and 7-o’clock positions; hemoclips are often used to control these bleeders. At the completion of this maneuver, hemostasis should be excellent.
-circle needle that was placed previously is used to oversew the superficial edges of the striated urethral sphincter–dorsal vein complex (Fig. 102–14). In placing this running suture, the surgeon should face the head of the table, holding the needle driver against the pubis perpendicular to the patient. If the needle driver is held loosely, the superficial edges of the complex can be easily approximated, forming a hood over the anterior urethra. Circumflex veins are often present at the posterior edge of the complex at the 5- and 7-o’clock positions; hemoclips are often used to control these bleeders. At the completion of this maneuver, hemostasis should be excellent.
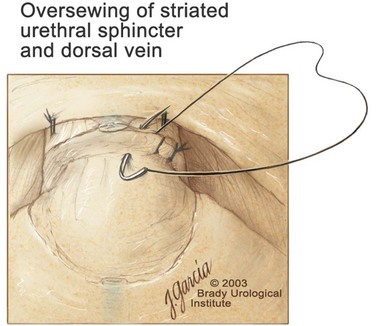
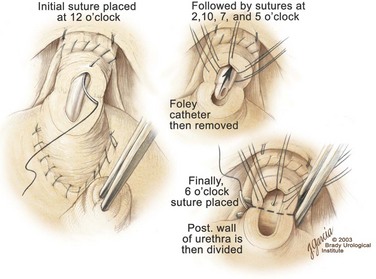
Division of the Urethra and Placement of the Urethral Sutures
 -circle tapered needle, the needle should incorporate just the urethral mucosa and submucosa but not the smooth muscle (Fig. 102–17). As stated previously, the surgeon should face the head of the table to place these sutures and should hold the needle driver against the pubis perpendicular to the patient. The first suture is placed in the mucosa and submucosa of the urethra at the 12-o’clock position. By use of a 16-Fr catheter, the mucosa is easily identified. The smooth muscle should not be incorporated in this stitch because stitches in the smooth muscle delay the recovery of urinary control. If the urethral tissue appears flimsy, this suture should then be placed through the dorsal vein complex to improve tensile strength. Once the mucosa and submucosa have been elevated by the 12-o’clock stitch, the remaining sutures are more easily placed. For a right-handed surgeon, it is easiest to place the 7- and 10-o’clock sutures from the outside of the lumen to the inside. The other three sutures are more easily placed beginning on the luminal surface and then proceeding outside. In performing the final anastomosis, a French eye needle can be used to place these three sutures through the lumen of the bladder. The sutures are covered with towels to avoid inadvertent traction or displacement.
-circle tapered needle, the needle should incorporate just the urethral mucosa and submucosa but not the smooth muscle (Fig. 102–17). As stated previously, the surgeon should face the head of the table to place these sutures and should hold the needle driver against the pubis perpendicular to the patient. The first suture is placed in the mucosa and submucosa of the urethra at the 12-o’clock position. By use of a 16-Fr catheter, the mucosa is easily identified. The smooth muscle should not be incorporated in this stitch because stitches in the smooth muscle delay the recovery of urinary control. If the urethral tissue appears flimsy, this suture should then be placed through the dorsal vein complex to improve tensile strength. Once the mucosa and submucosa have been elevated by the 12-o’clock stitch, the remaining sutures are more easily placed. For a right-handed surgeon, it is easiest to place the 7- and 10-o’clock sutures from the outside of the lumen to the inside. The other three sutures are more easily placed beginning on the luminal surface and then proceeding outside. In performing the final anastomosis, a French eye needle can be used to place these three sutures through the lumen of the bladder. The sutures are covered with towels to avoid inadvertent traction or displacement.
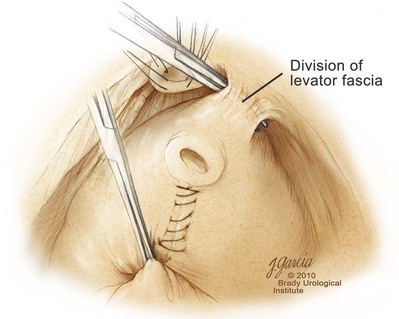
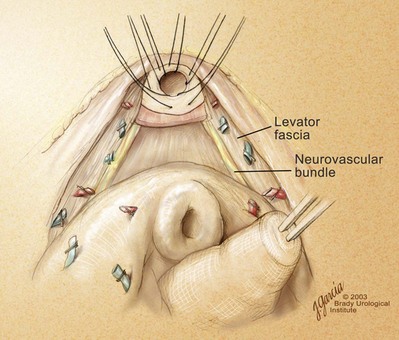
High Anterior Release of the Neurovascular Bundles at the Apex before Division of the Dorsal Vein Complex
Identification and Preservation or Wide Excision of the Neurovascular Bundles