17
Prostate Cancer: Controversies and Developments in Screening and Treatments
Case History
A 63-year-old Caucasian man had a routine physical examination by his family physician. He was told that the left lobe of his prostate was slightly firm. A prostate-specific antigen (PSA) and other routine blood work were done. On a return visit to his family physician he was informed that the PSA was 5.5 ng/mL, which is slightly high for his age. He was advised to undergo transrectal ultrasound guided prostate biopsy. Two weeks later he was asked to come to the doctor’s office with his wife. He was told that the biopsy was positive for prostate cancer. One of six core biopsies was positive for cancer. The tumor involved 3 mm in length of one core. The Gleason score was 3 + 3 = 6. He was then referred to a urologist for consultation. In the mean time he surfed the Internet and searched for information about prostate cancer. He was overwhelmed by the voluminous amount of information and confusing controversies regarding the treatment of prostate cancer.
Epidemiology
Prostate cancer is the most common male cancer and the second leading cause of cancer death in the United States. The American Cancer Society estimated that 220,900 men would be diagnosed with prostate cancer and 28,900 would die of the disease in 2003.1 Prostate cancer incidence and mortality vary markedly across geographic regions and populations. The prevalence of histological prostate cancer is similar among ethnic groups, while the incidence of clinically manifest disease differs markedly.2
The age-adjusted rates of prostate cancer in 1992 for African-American men and American white men were 249 per 100,000 and 182 per 100,000, respectively. The average age-adjusted incidence of prostate cancer in Kingston, Jamaica, is the highest in the world, 304 per 100,000.3 Asians, especially the Chinese, have the lowest incidence of prostate cancer, 28 per 100,000 for the Chinese and 39 per 100,000 for the Japanese.4 It appears that after migrating to the United States, however, the incidence rates for prostate cancer among Asians increase.5 African-American men have higher stage and grade of prostate cancer when it is diagnosed and the mortality of prostate cancer is higher than for American white men. The introduction of widespread PSA testing contributed to the dramatic increase in the incidence of prostate cancer between 1989 and 1991, and the incidence rates leveled off in 1992 as these prevalent cancers were detected. The mortality of prostate cancer has declined recently. However, because the natural history of prostate cancer is long, this decline in mortality cannot be attributed to widespread PSA testing.
Etiology of Prostate Cancer
The etiology of prostate cancer is multifactorial, and requires the interaction of various factors. Smoking has been associated with prostate cancer and so have the following factors, which should also be considered.
Age Factor
Age is an important factor. The prevalence of prostate cancer increases with age. The probability of developing prostate cancer is less than one in 10,000 in men aged 39 years or younger, one in 103 for men aged 40 to 59 years, and one in 8 for men 60 to 79 years.6
Genetic Factors
Ten to 15% of patients with prostate cancer have at least one relative who is affected, and first-degree relatives of patients with prostate cancer have a twofold or threefold increased risk of developing this disease. Men who have a brother who has prostate cancer are more likely to develop this disease than are those who only have a father who is affected, suggesting that the disease is recessive or linked to the X-chromosome.
Dietary Factor
Prostate cancer is positively associated with diet high in fat, meat, and dairy products. The low incidence of prostate cancer in the Asians might be related to consumption of diet with high content of phytoestrogens. Soybean has the highest contents of phytoestrogens and Asians consume soybean in large quantities.
Hormone Factors
Androgen plays an important role in the development and growth of prostate gland. Testosterone in the prostate cell is converted to dihydrotestosterone by 5α-reductase enzyme, which combines with the androgen receptor stimulating the prostate cell growth. Gann et al7 found that men with higher circulating levels of testosterone had an increased risk of prostate cancer compared with men with lower levels of testosterone. However, this association of prostate cancer with testosterone does not mean that testosterone if administered is causative of prostate cancer. Interestingly, men who were castrated before puberty or have congenital absence of 5α-reductase do not develop prostate cancer. A recent chemoprevention study has shown that 5α-reductase inhibitor reduces prostate cancer prevalence by 25%.8 This may support the hypothesis that dihydrotestosterone is associated with prostate cancer.
Principles of Screening Based on Epidemiological Principles
Does It Apply to Prostate Cancer?
In general, the purpose of screening is to find persons with risk factors in which preventive interventions could be used to identify individuals with early or asymptomatic treatable disease. This is in a sense an application of the principles of optimal aging, whereby the individual wants to minimize morbidity and mortality through preventive strategies after early detection. However, there are several principles that should guide a clinician as to whether it is worth screening in a population:
• The disease should be sufficiently common in the community because the detection of rare diseases may lead to high cost-benefit ratios. Prostate cancer is in no way a rare disease, and in fact it is the most common cancer in men. Each year ∼200,000 new cases of prostate cancer are diagnosed in the United States.9 Moreover, prostate cancer is a function of age, and prevalence rates increase as men age. Current statistics point toward a larger pool of baby boomers over the next few decades.
• The burden of suffering or the morbidity and mortality should be substantial. This is where screening can sometimes be seen as controversial. There is no doubt that this cancer is very common among aging men, but the morbidity and mortality from prostate cancer seem to vary from individual to individual. There may be also ethnic, dietary, and environmental factors that can determine the aggressiveness of these tumors. Many men die with prostate cancer rather than of prostate cancer. Often, complications from cardiovascular, pulmonary, infectious, and dementing disease cause the demise of older men, rather than prostate cancer itself. European experience, which includes watchful-waiting, has indicated that an aggressive approach to all patients with early disease would entail substantial overtreatment.10
• An effective preventive intervention or treatment should be available. Prevention of prostate cancer is very important. Lifestyle modifiable factors such as smoking, poor diet, and lack of exercise can influence the rates of prostate cancer. There have been some inroads in our knowledge of prostate cancer prevention recently. For instance, it was recently reported in a trial involving 18,882 men that finasteride might reduce the risk of prostate cancer.11 Finasteride is an inhibitor of 5α-reductase, and it inhibits the conversion of testosterone to dihydrotestosterone, which is the primary androgen in the prostate. In the Prostate Cancer Prevention Trial, it was reported that finasteride delays the appearance of prostate cancer, but the possible benefit and a reduced risk of urinary problems must be weighed against sexual side effects and the increased risk of high-grade prostate cancer. The role of selenium, vitamin E, zinc, and other supplements seem promising in preventing prostate cancer, but they have to be studied in greater detail, such as in the finasteride trial, before the widespread prescription of these supplements can be endorsed. The treatment of prostate cancer is discussed in more detail in subsequent sections, but it can be summarized under three options: radical prostatectomy, radiotherapy, and watchful waiting.
• The screening program should be acceptable and available for routine use in the general population. Here lies one of the biggest dilemmas in screening for prostate cancer. The most common test is the PSA. This test is neither very specific nor sensitive. For example, the PSA test is not very specific as only one third of men with an abnormal serum PSA level actually have cancer.12 Also, conditions such as prostatitis or even benign prostatic hyperplasia may even cause a rise in the PSA levels, sometimes causing false alarms. PSA as a serum marker is only 70 to 80% sensitive for prostate cancer. This serum marker is a protein made only by prostate cells. Serum PSA levels are proportional to either the total volume of prostate tissue or the amount of irritation in the prostate (such as occurs with carcinoma or inflammation). Either increased volume or irritation causes PSA to spill from the prostate into the bloodstream. Paradoxically, low levels of PSA can even indicate presence of cancers as well.13 Longitudinal studies in men suggest that PSA velocity of 0.7 ng per mL per year rise may be suggestive of cancer.14 Table 17–1 shows the relationship between percent PSA levels and the probability of prostate cancer.
Arguments for Screening
Prostate cancer is the most common cancer in men; as such, screening would help diagnose many of these patients in the early stage of their disease. Moreover, the American Cancer Society and the American Uro-logical Association recommend the use of a PSA-based screening program to detect prostate cancer in men 50 years of age and older.15 Some evidence also shows that, compared with screening by rectal examination alone, routine screening of asymptomatic patients with PSA testing and digital rectal examinations detects a higher percentage of cancers that are localized to the prostate.16 Although PSA alone may not be accurate enough, PSA density and PSA velocity may guide a clinician to a higher level of suspicion for this cancer. Smith et al17 determined, for the first 4 years of serial PSA-based screening, the trends in compliance, prevalence of abnormal screening test results, cancer detection rates, and stage and grade of cancers detected. In a community-based study of serial screening with PSA measurements, a total of 10,248 male volunteers at least 50 years old were screened at 6-month intervals for a minimum of 48 months. At 48 months, 79% of volunteers returned for screening. During this interval there was a decrease in the proportion of volunteers with serum PSA levels higher than 4.0ng/mL (from 10% to 6–7%), in cancer detection rates (from 3% to <1%), and in the proportion with clinically advanced cancer (from 6% to 2%). In men who underwent surgery, the proportion with high-grade cancer decreased (from 11% to 6%), and the proportion with pathologically advanced cancer was proportionately reduced but not significantly reduced. This study concluded that with serial PSA-based screening, the proportion of men with abnormal test results decreased, and the prostate cancer detection rate decreased to near the reported population-based incidence rate. There was also a shift to detection of cancers at an earlier clinical stage and detection of lower-grade cancers.
Arguments Against Screening
Some have argued that because prostate cancer screening does not fulfill all of the requirements for an effective screening program that it should not be performed routinely. Both the American Academy of Family Physicians and the U.S. Preventive Services Task Force recently recommended against the use of routine prostate cancer screening for two reasons: (1) early prostate cancer detection has no proven benefit; and (2) the potential side effects of treatment may outweigh the benefits.18
Although an individual PSA test is rather inexpensive, operating costs multiply when a patient with an abnormal PSA test must be evaluated. Transrectal ultrasound examination costs approximately $170 per patient, and random biopsies cost another $230. Pathologic evaluation of the biopsy specimens costs approximately $167 per patient. When compounded by the fact that three patients without cancer must be evaluated for each cancer that is detected, the estimated overall cost of initiating a nationwide prostate cancer screening and treatment program for all eligible men ranges from $8.5 to $25.7 billion per year.19
In all likelihood, many cancers that have been detected by PSA screening would never have become symptomatic in the patient’s lifetime. Many older men die of illnesses other than prostate cancer. Autopsy of elderly men often demonstrates prevalence rates of prostate cancers approaching 80%.20 However, the cause of death is often pneumonia, heart disease, and neurological disease. The natural history of prostate cancer is also not well defined, and there is great variation as to the aggressiveness of the tumor. A study in Sweden using watchful waiting rather than radical prostatectomy or hormonal therapy illustrates this point. Johnasson et al10 studied the natural history of initially untreated early-stage prostate cancer with a prospective cohort study; 642 patients with prostate cancer of any stage with a mean age of 72 years were studied. In the entire cohort, prostate cancer accounted for 201 (37%) of all 541 deaths. Among 300 patients with a diagnosis of localized disease (T0-T2), 33 (11%) died of prostate cancer. In this group, the corrected 15-year survival rate was similar in 223 patients with deferred treatment [81%; 95% confidence interval (CI), 72–89%] and in 77 who received initial treatment (81%; 95% CI, 67–95%). The corrected 15-year survival was 57% (95% CI, 45–68%) in 183 patients with locally advanced cancer (T3–T4) and 6% (95% CI, 0–12%) in those 159 who had distant metastases at the time of diagnosis. The Swedish study concluded that patients with localized prostate cancer have a favorable outlook following watchful waiting, and the number of deaths potentially avoidable by radical initial treatment is limited. Without reliable prognostic indicators, an aggressive approach to all patients with early disease would entail substantial overtreatment. In contrast, patients with locally advanced or metastatic disease may need trials of aggressive therapy to improve prognosis.
Controversies on Guidelines for Screening
Various professional organizations have variable screening recommendations:
• The American Cancer Society recommends that health care professionals should offer the PSA and digital rectal examination (DRE) yearly, beginning at age 50, to men who have at least a 10-year life expectancy. Men at high risk, such as African-Americans and men who have a first-degree relative diagnosed with prostate cancer at an early age, should begin testing at age 45.21
• The American Urological Association recommends that men older than 50 with a greater than 10-year life expectancy should be offered prostate cancer screening with PSA and DRE. Men at high risk should begin testing at age 45.22
• However, the U.S. Preventive Services Task Force (USPSTF) concludes that the evidence is insufficient to recommend for or against routine screening for prostate cancer using PSA testing or DRE.23
• The American College of Preventive Medicine recommends against routine population screening with DRE and PSA. Men age 50 or older with a life expectancy of greater than 10 years should be given information about the potential benefits and harms of screening and limits of current evidence of screening and should be allowed to make their own choice about screening, in consultation with their physician, based on personal preferences.24
• Whether screening for prostate cancer with early detection and treatment will be beneficial and reduce prostate cancer mortality will await the result of the ongoing large randomized control trials conducted by the United States Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial25 and the European Randomized Study for Screening for Prostate Cancer.26
Screening Tools Available to the Clinician
Digital Rectal Examination (DRE)
Historically, DRE is the first line of prostate cancer detection. Abnormal DRE, including in duration, irregular surface, asymmetry, nodule, and hardness, calls for prostate biopsy. DRE is subject to interpersonal and intrapersonal variation. DRE by itself is a poor screening tool. Despite the shortcomings of the DRE, up to 25% of prostate cancers are still detected by DRE in men with normal PSA levels. Therefore, a suspicious DRE should be followed by prostate biopsy.27 DRE when used with PSA together becomes a most effective tool for detecting prostate cancer in its earliest stages; 50% of men with abnormal DRE and PSA were found to have prostate cancer on biopsy.28
Prostate Specific Antigen (PSA)
PSA is a serine protease produced by the epithelial cells of the prostate and the periurethral glands. It has a half-life of 3 days. It is the best tumor marker. When comparing PSA to mammography in terms of cancer detection, an abnormal PSA of a man over the age of 50 is twice as likely to detect cancer than is an abnormal mammogram in a woman over the age of 50. PSA is not prostate cancer specific, however. Normal prostate gland, benign prostate hyperplasia, prostatitis, urinary tract infection, riding a bicycle in the previous 48 hours, and sexual intercourse with ejaculation within 48 hours will increase the serum levels of PSA. The normal range of PSA is 0 to 4.0 ng/mL. The sensitivity, specificity, and positive predictive value of PSA is 79%, 59%, and 40%, respectively.29 Using PSA 4ng/mL as a cutoff, 25% of men with benign prostate hyperplasia (BPH) have a PSA >4 ng/mL and 25% of men with prostate cancer have a PSA <4ng/mL. The low specificity of PSA creates false positivity, resulting in unnecessary prostate biopsy, patient’s anxiety, and medical cost. To improve the predictive value of PSA for cancer, it is recommended that the levels of PSA be adjusted for age (age-specific PSA) and for prostate volume (PSA density, PSAD), and be evaluated for the rate of change (PSA velocity). Recently, the molecular forms of PSA in free and bound fractions in the serum were quantified. The measurement of free and bound PSA has been evaluated to differentiate BPH from cancer. The age-specific range of normal PSA includes < 2.5ng/mL for age 40 to 49; <3.5ng/mL for age 50 to 59; <4.5ng/mL for 60 to 69; and <6.5ng/mL for age 70 to 79. When PSAD is >0.15 and PSA is 4 to 10ng/mL with a normal DRE, prostate biopsy is recommended. The rate of change of PSA >0.75ng/mL per year is significant to warrant a prostate biopsy. It has been shown that men with prostate cancer have a greater fraction of PSA bound to α-antichymotrypsin and a lower percentage of PSA that is free. If the ratio of free PSA to total PSA is <25% in men with total PSA between 4 and 10 ng/mL and if the prostate is normally palpable, 95% of prostate cancers were detected and 20% of unnecessary biopsies were avoided.30
Transrectal Ultrasonography (TRUS)
Studies have confirmed that TRUS cannot localize early prostate cancer.31 The major role of TRUS is to biopsy the suspicious area and do a systemic biopsy of the prostate gland.
Beyond Prostate Specific Antigen
Since 1998, the “free” or “unbound” PSA test has been Food and Drug Administration (FDA)-approved to augment information available from the total PSA. The “free” PSA test is used as a ratio with the total PSA to help give a more precise determination of the risk of prostate cancer. In essence, the lower the amount of free PSA, the higher the likelihood of cancer. The free PSA test is often used following a nonsuspicious DRE and a total PSA test that shows moderately elevated PSA levels (between 4 and 10 ng/mL) in men aged 50 years and older.
In addition, two novel molecular forms of “free” PSA exist. BPSA and proPSA are distinct molecular forms of free PSA in serum. BPSA (benign PSA) and is more often associated with benign enlargement of the prostate gland. On the other hand, the inactive precursor of PSA, or truncated proPSA, is more often associated with cancer. ProPSA is composed of native proPSA as well as two truncated proPSAforms, [–2]pPSA and [–4]pPSA, and both have been shown to be more cancer-associated. Preliminary studies indicate that BPSA is a biomarker for clinical BPH. Early studies also reveal that truncated proPSA significantly increases the specificity for prostate cancer especially in the 2- to 4-ng/mL PSA range.32 It is estimated that as many as 40% of prostate cancer patients are within the 2- to 4-ng/mL total PSA range. Further studies are currently underway to determine the potential range and application of clinical utility of truncated proPSA in prostate cancer detection and management. These new tests may further enhance the physician’s ability to differentiate between prostate cancer and benign disease in men with slightly elevated PSA levels.
Other exciting possibilities for more precise prostate cancer screening include the TGG β growth factor blood test and the IL-6 soluble receptor blood test that can help distinguish between more and less aggressive forms of prostate cancer. Shariat et al33 reported that plasma interleukin-6 (IL-6) and IL-6sR levels were dramatically elevated in men with prostate cancer metastatic to bone. In patients with clinically localized prostate cancer, the preoperative plasma IL-6 and IL-6sR levels independently predicted biochemical progression after surgery, presumably because of an association with occult metastatic disease present at the time of radical prostatectomy. Another test that is being explored is the human glandular kallikrein-2.34
Prostate Biopsy and Grading of Prostate Cancer
The Gleason grading system is the most commonly used histological grading system for prostate cancer. It is based on the architectural pattern, the primary predominant pattern, and the secondary most predominant pattern. Both patterns are assigned a grade from 1 to 5. Pick the two most predominant patterns, for example, 3 and 4. Add the most predominant patterns to arrive at the Gleason score, for example, 3 + 4 = 7. If only one pattern predominates, double this pattern, for example, 3 + 3 = 6. The Gleason scores are grouped according to the following ranges: 2 to 4, 5 to 6, 7, and 8 to 10. The Gleason score correlates with prognosis after radical prostatectomy. A Gleason score 7 tumor behaves significantly worse than Gleason score 5 to 6 tumors but fare better than Gleason score 8 to 9 tumors. The biopsy Gleason score can also be combined with serum PSA values and clinical stage to predict organ-confined versus non-organ-confined disease and the risk of progression after radical prostatectomy.35,36 Like the DRE, there is interobserver variation in assigning Gleason score.
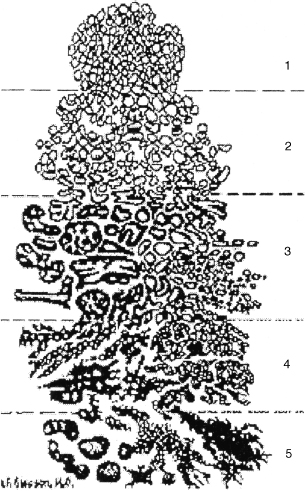
FIGURE 17-1. Histological guide to Gleason score.
Recently, Lattouf and Saad37 set out to assess the correlation of the Gleason score on the initial prostate biopsy and the final pathology after radical prostatectomy (RP) for prostate adenocarcinoma. Often there was poor correlation with the prostate biopsy reading and the final pathological report after surgery. As such, the investigators concluded that Gleason grading of the prostate biopsy could be a poor predictor of pathological outcome. Assessment by the same pathologist may reduce the discrepancy. The authors suggest that clinicians should be aware of these limitations when using the biopsy Gleason grade in decision making. Fig. 17–1 summarizes the histological Gleason grading.
Staging of Prostate Cancer
The tumor-node-metastasis (TNM) classification system is the most common system used to evaluate local and distant extent of disease for the staging of prostate cancer. T1 is clinically localized tumor not palpable on DRE. T1a is focal tumor [<5% of resected tissue on transurethral resection of the prostate (TURP)] and low grade. T1b is diffuse tumor >5% of resected tissue on TURP or high grade. T1c is the tumor diagnosed based on elevated PSA and not palpable on DRE. T2 tumor is clinically localized, tumor palpable. T2a tumor involves half a lobe or less; T2b tumor involves more than half a lobe; T2c tumor involves both lobes; T3 is locally invasive beyond the prostatic capsule and tumor is palpable. T3a is unilateral extracapsular extension; T3b is bilateral extracapsular extension; and T3c is seminal vesicle invasion. T4 tumor invades adjacent tissues (e.g., bladder, rectum, levator ani muscles), and N/M indicates the metastatic disease. N1 is microscopic pelvic lymph node metastasis; N2 is gross pelvic lymph node metastasis; and N3 is extrapelvic lymph node metastases. M is distant metastases (lung, bones, liver, and brain). Staging prostate cancer depends on the DRE finding. There is interpersonal variation, in that two examiners examining the same patient may have two different findings.
Treatment of Prostate Cancer
Successful treatment of prostate cancer depends on patient selection. The use of PSA testing combined with DRE has revolutionized our ability to detect prostate cancer at its early and curable stage. However, not all patients with prostate cancer require treatment. According to the study by Albertsen et al,38 in 4 to 7% of patients with a Gleason score of 2 to 4, 6 to 11% of patients with a Gleason score of 5, 18 to 30% of patients with a Gleason score 6, 42 to 70% of patients with a Gleason score 7, and 60 to 87% of patients with a Gleason score of 8 to 10, prostate cancer progressed to cause death in 15 years. The majority of patients diagnosed with prostate cancer are 65 years and older, when they may have many other comorbidities that may compete with prostate cancer to cause death. The candidate for treatment of clinically localized prostate cancer with curative intent should have a life expectancy of 10 to 15 years. Clinically localized prostate cancer can be treated with radical prostatectomy, external beam radiation therapy, brachytherapy, or conservative therapy (watchful waiting) with acceptable good result. A recent randomized control trial from Sweden has shown that the cancer-specific mortality is significantly reduced in the radical prostatectomy group versus watchful-waiting group, though the overall survival is no different.39 However, these patients’ diagnosis of prostate cancer is clinical based rather than PSA based. There is no randomized control trial among radical prostatectomy, external beam radiation therapy, and brachytherapy to compare the superiority of these treatment modalities. Therefore, the choice among radical prostatectomy, external beam radiation, or brachytherapy is hard to make both for physician and patient.
Radical Prostatectomy
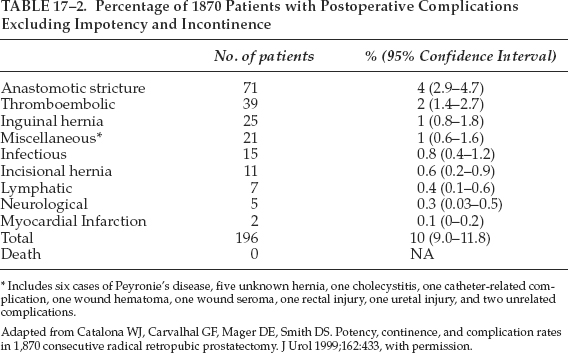
Radical prostatectomy is performed through either the retropubic or perineal approach. Most urologists are trained in retropubic prostatectomy. Radical retropubic prostatectomy is an effective modality for treatment of clinically localized prostate cancer. The mortality rate of radical prostatectomy has been low and approaches zero in recent series (Table 17–2).40 Excellent long-term results can be obtained with radical retropubic prostatectomy. The Johns Hopkins Hospital group reported that in 2404 men who underwent radical retropubic prostatectomy for clinically localized prostate cancer, the overall actuarial PSA progression-free survival at 5, 10, and 15 years were 84%, 74%, and 66%, respectively. The actuarial metastasis-free survival at 5, 10, and 15 years were 96%, 90%, and 82%, respectively, and the actuarial cancer-specific survival at 5, 10, and 15 years were 99%, 96%, and 90%, respectively.41 The improvement of knowledge of surgical anatomy with discovery of the dorsal venous complex around the apex of the prostate42 and the cavernous nerve in the path of the capsular branch of the inferior vesical artery43 has reduced the intraoperative blood loss, the perioperative morbidity, and increased the rate of continence and potency preservation of radical retropubic prostatectomy. Radical perineal prostatectomy has the advantages of less morbidity, much less blood loss and easier access to the vesicourethral anastomosis, and shorter hospital stay. However, it is believed that radical perineal prostatectomy creates more positive margins of greater extent than does the radical retropubic prostatectomy.44
Complications of Radical Retropubic Prostatectomy
INTRAOPERATIVE COMPLICATIONS
Blood loss can average 1000 mL45 and is replaced by allogenic or autologous blood transfusion. Cell salvage can be used as well. It is a safe method of transfusion during radical retropubic prostatectomy.46 Rectal injury with an occurrence rate of 0 to 5.3%47 can be repaired by the two-layer method without colostomy, provided that preoperative bowel preparation has been performed. Ureteral injury rates are 0 to 1.6%47 and can be repaired with placement of a double J-stent. Obturator nerve injury, very uncommon during pelvic lymphadenectomy if recognized can be repaired primarily, though the long-term functional status after repair is not available.
PERIOPERATIVE COMPLICATIONS
Like any other major surgery in the older patient, radical retropubic prostatectomy may be associated with medical complications such as pneumonia, atelectasis, pulmonary embolism, deep vein thrombosis, myocardial infarction, and postoperative ileus. Early surgical complications include wound infection, wound dehiscence, delayed bleeding, catheter dislodgment, anastomotic leakage, and lymphocele. Hedican and Walsh48 reported that 7 out of 1350 patients undergoing radical retropubic prostatectomy (0.5%) developed delayed bleeding requiring acute blood transfusion, and four who were managed by exploration had good outcomes. Catheter dislodgment before 3 or 4 days probably may require replacement of a new catheter using a flexible cystocope with a guide wire. Leaking urine from the urethrovesical anastomosis occurs in from 0.1 to 21.1%, and spontaneous resolution usually results in prolonged catheterization.47 Lymphocele may occur after radical retropubic prostatectomy with bilateral pelvic lymphadenectomy. It may require percutaneous drainage with ultrasound guidance or open drainage with marsupialization for persistent lymphocele.
LONG-TERM COMPLICATIONS
The long-term complications of radical retropubic prostatectomy can include urinary incontinence, erectile dysfunction, and bladder neck contracture.
Urinary Incontinence
Urinary incontinence is a devastating complication of radical prostatectomy. The definition of incontinence is not universal. This leads to different incidence rates of incontinence after radical retropubic prostatectomy. The Johns Hopkins Hospital group treated 593 consecutive patients who had 92% complete continence, with some degree of stress incontinence in 8%.49 The American College of Surgeons Committee on Cancer surveyed 1796 preoperatively continent men who had undergone radical retropubic prostatectomy at 484 hospitals and found that 19% had incontinence and required pads daily and 3.6% had total incontinence.50 Minor incontinence or stress incontinence can be managed by pads or a Cunningham clamp. Severe or total incontinence may require artificial urethral sphincter placement.
Erectile Dysfunction
Erectile dysfunction was universal after radical retropubic prostatectomy before Walsh and Donker43 in 1982 discovered the autonomic branches of the pelvic plexus innervating the corpora cavernosa and developed techniques to preserve them. Preservation rates of erectile function after radical retropubic prostatectomy vary widely. Quinlan et al51 reported the potency rate in 600 patients who underwent the nerve-sparing approach. Of 503 patients who were potent preoperatively, 68% were potent postoperatively. Age, stage of the disease, and either unilateral or bilateral nerve-sparing procedure were significant factors for preservation of potency. Potency was preserved in 91% of men aged less than 50 year, whereas it was only 25% of men aged 70 years or older. The potency rates in younger patients were better when bilateral nerve-sparing procedures were performed. However, when nonsurgical members of the health care team questioned patients about their sexual function, potency rates were preserved in 31.9% and 13.3% of bilateral and unilateral nerve-sparing procedures, respectively.52 The cavernous nerve innervating the corpora cavernosa is a microscopic structure and the capsular branch of the inferior vesical artery is not readily identified during the radical retropubic prostatectomy. A device, the CaverMap Surgical Aid (UroMed, Boston, MA), was designed to aid the surgeon in identifying and preserving neurovascular bundles (NVBs). However, the size of the CaverMap nerve stimulator may make it difficult to trace the cavernous nerves before the prostate is removed, particularly in obese men or in patients who have a large prostate or a narrow pelvis. In a randomized, controlled study, the use of the CaverMap during radical prostatectomy resulted in improved nocturnal erections, but did not lead to improved overall sexual function.53 Sural nerve graft during radical retropubic prostatectomy may enhance the recovery of erectile function when the neurovascular bundles were resected.54 The importance of nerve grafting is uncertain.55 Despite nerve-sparing procedure recovery of erectile function may take months to 2 years. Therapy may be necessary for some patients to restore the erectile function in the interim. There are various means that can restore erectile function. Zippe et al56 reported that sildenafil (Viagra) helped men who had undergone bilateral nerve-sparing radical retropubic prostatectomy to obtain erections sufficient for intercourse, whereas no men who had undergone excision or ligation of both neurovascular bundles had a positive response to this agent. Intracavernous injection with vasoactive agents such prostaglandin E1 is effective to achieve sufficient erection for vaginal intercourse more than 90% of the time.57 Transurethral application of prostaglandin E1 may help patients achieve erection 64.9% of the time.58 Using a vacuum erection device can help men achieve an adequate erection. Penile prostheses, either inflatable or semirigid, are the ultimate solution to erectile dysfunction after radical retropubic prostatectomy.
Bladder Neck Contracture
The incidence of bladder neck contracture ranged from 1.3 to 27% in early studies.59 The scarring at the site of the urethrovesical anastomosis was thought to result from the extravasation of urine. With eversion of the bladder neck mucosa and watertight urethrovesical anastomosis, the bladder neck contracture rates have reduced. Poon et al60 reported that there was no statistical difference with regard to bladder neck contracture rates whether the urethrovesical anastomosis is achieved by bladder neck preservation, tennis racket type closure, or anterior bladder tube bladder neck reconstruction. Bladder neck contracture may present clinically as urinary incontinence or obstruction. The treatment is bladder neck dilation, incision, or resection.
External Beam Radiation Therapy (EBRT)
Prostate cancer is a radiosensitive tumor. The higher the radiation dose delivered to a given volume of cancer, the more likely that the cancer will be permanently controlled. External beam radiation therapy is usually administered in once-daily fractions, 5 days per week over a period of 7 to 8 weeks. Each treatment session lasts ∼10 to 20 minutes.
Conventional External Beam Radiation Therapy
The 10-year disease-specific survival of 1557 prostate cancer patients treated with conventional external beam radiation therapy alone was 87%, 75%, and 44% for tumors of Gleason score 2 to 5, 6 to 7, and 8 to 10, respectively.61 PSA failure definitions after EBRT vary from >0.5 to >4ng/mL among radiation oncologists. The American Society for Therapeutic Radiology and Oncology recommends that PSA failure definition is three consecutive increases in PSA from the nadir value. The date of failure should be the midpoint between the nadir and the first of the three PSA rising values, and PSA testing should be performed every 3 to 4 months.
Zietman et al62 reported that less than 50% of the T1- or T2NxM0 and less than 20% of the T3- or T4NxM0 prostate cancer patients receiving conventional radiation therapy were biochemical disease free at 10 years. Conventional external beam radiation therapy irradiated large volumes of normal tissues as well as the prostate gland. To avoid late side effects such as rectal bleeding, the radiation dose is limited to 60 to 70Gy, delivered in 2-Gy daily fractions.
Three-Dimensional Conformal Radiation Therapy (3D-CRT)
With the development of three-dimensional conformal radiation therapy (3D-CRT), a higher radiation dose can be delivered to the tumor target improving treatment efficacy while reducing the radiation to the normal tissues and avoiding late side effects. Study of patients treated with 3D-CRT with tumor target dose increasing from 64.8 to 81Gy was reported. PSA nadir ≦1.0ng/mL was achieved in 90% of the patients treated with doses 75.6 or 81 Gy, in 76% of those treated with 70.2 Gy, and in 56% of those treated with 64.8 Gy. The 5-year PSA relapse-free survival rate was 85% for patients with a good prognosis, 65% for intermediate prognosis, and 35% for unfavorable prognosis. A positive biopsy at >2.5 years after 3D-CRT was observed in only 1/15 (7%) of patients receiving 81.0Gy, compared with 12/25 (48%) after 75.6Gy, 19/42 (45%) after 70.2 Gy, and 13/23 (57%) after 64.8Gy (p<.05). This study provides evidence for a significant effect of dose escalation on the response of human prostate cancer to irradiation.63 The GU Radiation Oncologists of Canada recommends classification of prostate cancer into three groups based on prognostic grouping and risk of recurrence: (1) low-risk group with PSA ≦10ng/mL, Gleason score ≦6 and stage T2a or less; (2) intermediate-risk group with PSA ≦20ng/mL, Gleason score <8, and stage T1/T2; and (3) high-risk group with PSA >20ng/mL, Gleason score ≥8 and stage ≥T3a. Conformal treatment techniques should be offered to patients receiving prostatic radiation therapy. Low-risk patients receive 70 Gy in 2-Gy fractions, intermediate-risk patients receive 75 to 78 Gy in 2-Gy fractions, and the high-risk patients are at high risk of systemic metastases. It is unclear how dose escalation using local therapy would improve outcome. It was felt that conformal doses in the range of 75 to 78 Gy is a reasonable option for high-risk group patients.64
Intensity Modulated Radiotherapy
Recently, it has become possible not only to shape the borders of the radiation fields to conform to the target volume, but also to define spatial variation in beam intensity within each field. This technique, known as intensity modulated radiotherapy (IMRT), enables the high-dose radiation envelope to form complex shapes, providing even greater conformality of radiation dose to the target volume. It is therefore expected to further reduce the risk of normal tissue complications, and so enable greater dose-escalation. At New York Memorial Sloan-Kettering Cancer Center, a total of 698 patients (90%) were treated with 81.0 Gy, and 74 patients (10%) were treated with 86.4 Gy. Using the IMRT technique for clinically localized prostate cancer, the 3-year rate of grade ≥2 rectal toxicity is just 4%.65
Complications of External Beam Radiation Therapy
The conventional techniques of external-beam radiation therapy are fairly well tolerated, although grade 2 or higher acute rectal morbidity (discomfort, tenesmus, diarrhea) and/or urinary symptoms (frequency, nocturia, urgency, dysuria) requiring medication occur in ∼60% of patients. The incidence of late complications is low. An analysis of 1020 patients treated in two large Radiation Therapy Oncology Group (RTOG) trials66 demonstrated an incidence of chronic urinary sequelae (i.e., cystitis, hematuria, urethral stricture, or bladder contracture) requiring hospitalization in 7.3% of cases, but the incidence of late urinary toxicity requiring major surgical intervention was only 0.5%. Urethral stricture is more common in patients who had undergone a previous TURP. The incidence of chronic intestinal sequelae (chronic diarrhea, proctitis, rectal or anal stricture, rectal bleeding, or ulcer) requiring hospitalization for diagnosis and minor intervention was 3.3%, with 0.6% of patients experiencing bowel obstruction or perforation. Fatal complications were extremely uncommon (0.2%).66 Most adverse events are observed within the first 3 to 4 years after treatment; the likelihood of complications developing after 5 years is low.67 The risks of complications are increased when radiation doses exceed 70Gy68 The risk of rectal toxicity has been correlated with the volume of the anterior rectal wall exposed to the higher doses of irradiation.69 Erectile potency appears to diminish with advancing time after treatment due to vascular disruption from radiation, with half of patients impotent at 7 years after irradiation.70
Prostate Brachytherapy
Prostate brachytherapy is a technique whereby small radioactive implants or seeds are placed within the prostate gland, allowing high radiation dose to tumors (Fig. 17–2). In the 1970s, Whitmore and colleagues71 developed the technique of open retropubic approach and free-hand implantation of iodine 125 seeds in the treatment of prostate cancer. The poor dose distribution and the inclusion of patients with locally advanced disease led to poor treatment result. The technique was abandoned.72 Follow-up of Whitmore et al’s patients showed a biochemical diseased free rate of only 13% at 15 years.73 Accurate transperineal placement of radioisotope seeds through a template with real-time transrectal ultrasound guidance developed by Holm et al74 began the modern era of prostate brachytherapy in 1983. Blasko and colleagues75 developed and improved the technique with reduced complication and improved outcomes. Iodine 125 (I-125) and palladium 103 (Pd-103) are the commonly used isotopes for prostate brachytherapy. The half-life of I-125 is 60 days and Pd-103 17 days. Based on the consideration that the effective dose of radiation is largely delivered in the first three half-lives, then the duration of treatment can be estimated at 180 days for I-125 and 51 days for Pd-103.76 The recommended dose for monotherapy is 145 Gy for I-125 and 125Gy for Pd-103. Pd-103 was preferred for higher grade lesions, though there is no proven data to suggest that Pd-103 is superior to I-125 or vice versa. There are three phases of permanent prostate brachytherapy: the planning phase, the implant procedure, and implant quality evaluation.
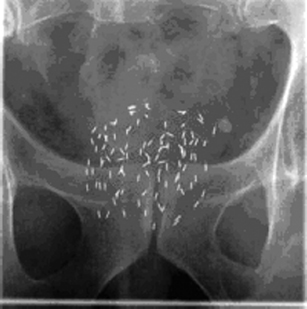
FIGURE 17-2. Brachytherapy with radioactive implants.
During the planning phase the prostate volume is determined by transrectal ultrasound. The ultrasound image of prostate volume is digitalized into the planning computer to generate a treatment plan with the desired dose of radiation delivering to the prostate while assuring that neither the rectum nor the urethra receives excessive radiation. The planning phase can be performed intraoperatively prior to implant procedure. The implant procedure is done through real-time ultrasound-guided transperineal percutaneous implantation on an outpatient basis. The implantation is done in the operating room under spinal or general anesthesia. The procedure usually lasts 45 to 60 minutes. Implant quality evaluation by calculation of the radiation doses can be assessed by radiographs. This method does not provide information relative to the prostate location.77 Computer tomography (CT)-based dosimetry methods have been recommended to assess implant quality by the American Brachytherapy Society.78 According to the most recent analysis from the Seattle brachytherapy data, the 10-year PSA progression-free rate following brachytherapy for low-risk prostate cancer was 87%, intermediate-risk 79% and high-risk 51%.79 Hence, favorable result from prostate brachytherapy is obtainable only in low-risk patients. The American Brachytherapy Society currently recommends prostate monobrachytherapy for clinical T1-T2, Gleason score ≦6 and PSA ≦10ng/mL disease, that is, low-risk tumor. High-risk patients are recommended to be treated with combination of brachytherapy and EBRT.78 Ragde80 reported 12-year observed follow-up on patients treated with brachytherapy alone for the low-risk group and combined EBRT with brachytherapy for the high-risk group. It is interesting that the 12-year PSA progression-free rate was 66% for the low-risk group and 79% for the high-risk group. The rate might have been better than 66% had the low-risk group been treated with a combination of EBRT and brachytherapy. Androgen deprivation therapy to downsize the prostate gland to less than 60 cc may be necessary to avoid pubic arch interference during seed implantation. Luteinizing hormone-releasing hormone (LHRH) agonist with or without antiandrogen is commonly used for androgen deprivation.
Complications of Prostate Brachytherapy
Complications of prostate brachytherapy reported from 13 case series and three cohort studies included acute urinary retention (1–14%), incontinence (5–6%), cystitis/urethritis (14%), urethral stricture (1%), proctitis (1–14%), and impotence (4–50%).81
Cryotherapy
The availably of real-time transrectal ultrasound monitoring of the cryoprobe placement, the development of 3-mm cryoprobes that could be placed transperineally into the target areas of the prostate, and the development of urethral warming systems to maintain the periurethral tissues viability have made cryotherapy of prostate cancer feasible. Five or six cryoprobes delivering liquid nitrogen to achieve a temperature of –40° to –50°C at the periphery of the prostate gland are necessary to obtain coagulative necrosis. In addition four or five thermocouple probes are placed to ensure an adequate freeze. The urethral warming catheter systems are necessary to minimize urethral sloughing during cryotherapy. The role of prostate cancer cryotherapy, according to Benoit et al,79 is principally in the treatment of high-risk clinically localized prostate cancer. It is also the treatment of choice for men with local failure after EBRT. There is no 10-or 15-year result as measured by PSA progression. Five-year actuarial biochemical-free survival post-cryotherapy was 51% and 63% for PSA less than 0.5ng/mL and PSA less than 1ng/mL, respectively.82
Complications of Cryotherapy
Complications of cryotherapy include dramatic scrotal edema, though resolving spontaneously within 3 weeks, urethral sloughing 15%, urinary incontinence 5.9%, urethral stricture 4.9%, bladder neck contracture 3.2%, perineal pain 0.6%, urethrorectal fistula 0.3%, sepsis 0.8%, urinary retention 1.2%, and stress incontinence 0.3%.79
Other Ablative Methods
HIGH-INTENSITY FOCUS ULTRASOUND
High-intensity focus ultrasound (HIFU) delivers intense ultrasound energy, with consequent heat destruction of tissue at a specific focal distance from the probe without damage to tissue in the path of the ultrasound beam. A piezoelectric transducer placed transrectally emits a highly focused convergent ultrasound beam in pulses lasting 3 to 5 seconds. These pulses produce areas of ablation that are ellipsoid in shape and typically measure ∼2 cm in height by 2mm in diameter. Temperatures in the target area range between 85° and 100°C, high enough to produce discrete areas of coagulative necrosis. The transducer is moved sequentially through various areas of the prostate to produce large areas of focal ablation.83 The procedure is done under general or spinal anesthesia. HIFU can be performed for patients with localized prostate cancer without an incision, with a less severe side-effect profile, and, unlike most other prostate treatments, is repeatable.84 Long-term PSA result is lacking. Further study with this technology is warranted.
RADIOFREQUENCY INTERSTITIAL TUMOR ABLATION (RITA)
In this procedure, radiofrequency electrodes are placed under sonographic guidance transperineally into target areas of the prostate, and large spherical areas of coagulative necrosis can be produced. Treatment time is short (8 to 12 minutes). Various size lesions can be generated.
Interstitial Thermal Ablation
In this procedure, specially derived cobalt-palladium alloy seeds are placed in the target area (i.e., the prostate) under transrectal sonographic guidance. The seeds are 1 mm in diameter by 14 mm in length and will self-regulate to desired temperature when placed in a magnetic field. Neither RITA nor interstitial thermal ablation has generated enough peer-review clinical information as treatment options for prostate cancer.82
Androgen Deprivation and Prostate Cancer
Huggins and Hodges85 described androgen sensitivity of prostate cancer in 1941. Androgen deprivation induces apoptosis and inhibition of cell proliferation of the prostate cancer tissues.
Neoadjuvant Hormone Therapy
Androgen deprivation used prior to primary treatment of prostate cancer is called neoadjuvant hormone therapy (NHT). NHT 3 to 8 months prior to radical prostatectomy may reduce margin positivity by 50%. It is hoped this will translate into improved disease-free survival. However, studies have shown no difference in disease-free survival with or without NHT up to 5 years of follow-up. Until recurrence rates have been demonstrated to decrease in randomized studies, NHT should be considered investigational and studied in the context of controlled clinical studies.86
In high-risk patients, NHT followed by EBRT has been shown to improve biochemical disease-free and metastasis-free survival versus EBRT alone.87 NHT is used prior to brachytherapy to downsize the prostate gland to less than 60 cc to avoid pubic arch interference. Currently there is no evidence that the addition of androgen deprivation to brachytherapy will improve the biochemical disease-free survival.77
Adjuvant Hormone Therapy
Androgen deprivation used following primary treatment of prostate cancer when the disease fails to be completely eradicated is called adjuvant hormone therapy (AHT). The therapy is usually started within 6 months of the primary treatment. A report by Messing et al88 showed biochemical or clinical progression and overall survival advantage when immediate adjuvant hormone therapy was given to the patients with positive pelvic nodes after radical prostatectomy versus observation alone. The most convincing data favoring the use of androgen deprivation therapy in conjunction with EBRT were reported by Bolla et al.89 A significant difference in overall survival was noted for patients who received adjuvant hormone therapy with radiation when compared with those who underwent radiation therapy alone. Five-year overall survival for the two groups was 79% and 62%, respectively.89
Rising Prostate-Specific Antigen After Primary Treatment
At least 35% of men who received primary therapy for clinically localized prostate cancer with curative intent will experience PSA failure within 10 years.90 The management of rising PSA after radical prostatectomy or radiotherapy is an increasingly common problem facing patients and physicians. The objectives of treating a rising PSA level are to prevent metastasis, symptoms, or death due to prostate cancer. Pound et al91 reported the outcomes of 1997 men who underwent a radical prostatectomy over a 15-year period. The median follow-up was 5.3 years. At the time of analysis, 315 patients’ disease had recurred as shown by detectable PSA. The importance of this study is that it provides evidence that a rising PSA after radical prostatectomy does not mean a death sentence for all patents. It pointed out that for Gleason score 5 to 7 prostate cancer, if the rising PSA recurred in 2 years or more after surgery and PSA doubling time was 10 months or more, the probability of metastatic progression was 5%, 14%, and 18% in 3, 5, and 7 years, respectively. The median time to metastatic progression was 8 years, yet 63% of the patients with rising PSA remained free of metastasis at 5 years. Once the metastatic disease was documented, the median time to death was 5 years.91 Rising PSA after surgery can be effectively treated with salvage EBRT if there is no distant disease. Androgen deprivation therapy may be considered in patients at high risk of metastatic progression. The incidence of biochemical failure after EBRT or brachytherapy is at least as high as after radical prostatectomy.
In Critz et al’s92 study, in which a combination of brachytherapy and EBRT was administered to 660 men with T1–T2 disease, patients who achieved a PSA nadir of ≦0.5ng/mL had a 5-year disease-free survival of 93%, whereas only 26% of patients in whom the nadir was ≥0.6ng/mL were disease-free at 5 years. All men with a nadir of >1ng/mL eventually failed treatment. The authors concluded that a PSA nadir of ≦0.5 ng/mL should be reached for a good prognosis. However, this value is not accepted by all radiation oncologists. Patients who failed after EBRT can be treated with salvage radical prostatectomy, cryotherapy, or brachytherapy if the site of recurrent disease is considered to be local rather than distant. Salvage surgery is feasible but is associated with significant morbidity. Short-term follow-up of salvage cryotherapy has shown reduction of PSA, though with high urinary obstruction required transurethral surgery.93 Salvage brachytherapy has significant risk of incontinence. The majority of patients with rising PSA after EBRT are managed by androgen deprivation therapy.
Method of Androgen Deprivation Therapy
ORCHIECTOMY
Androgen deprivation therapy can be surgical or medical. Bilateral orchiectomy is a simple, safe, and low-cost surgical procedure.94 For decades this has been the most common and effective treatment for metastatic prostate cancer. Serum testosterone levels were reduced by 95% within 3 hours of castration. Therefore, some patients may suffer psychological trauma from an orchiectomy.
ESTROGEN
Estrogen in the form of diethylstilbestrol 3 to 5mg daily reduces the testosterone secretion from the Leydig cells of the testes to castration levels by downregulating the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) by the pituitary gland. This is achieved at between 3 and 9 weeks.95 Diethylstilbestrol may cause direct cytotoxic effects on prostate cancer cells by cytoplasmic microtubules disruption.96 The side effects of diethylstilbestrol include nausea, vomiting, gynecomastia, fluid retention, and cardiovascular events.
ANTIANDROGEN
There are two classes of antiandrogen: steroidal and nonsteroidal. Steroidal antiandrogens such as cyproterone acetate and megestrol acetate inhibit c21–9 decarboxylate, preventing adrenal androgen synthesis and gonadotropin release from the hypothalamus. Nonsteroidal antiandrogens include flutamide, bicalutamide, and nilutamide. They block the androgen receptor from binding to dihydrotestosterone and testosterone. The serum levels of testosterone will increase. Flutamide may induce gastrointestinal and liver toxicity, whereas bicalutamide has fewer of these side effects. Nilutamide may cause flushing, poor visual adaptation to dark, and pulmonary fibrosis. Monotherapy using bicalutamide 150 mg daily compared with orchiectomy or LHRH agonist has been shown to have equivalent survival with an improvement of sexual function and physical capacity for those patients treated with bicalutamide.97
LUTEINIZING HORMONE-RELEASING
HORMONE (LHRH) AGONISTS
Medical castration can be effected by inhibition of LH secretion by giving LHRH-agonist injection subcutaneously. Lupron (leuprolide acetate), Zoladex (goserelin acetate), and Suprefact (buserelin) are LHRH agonists, available for injection every 3 months. Initially the LHRH agonists stimulate secretion of LH and FSH from the pituitary gland. The LH promotes testosterone production from the Leydig cells of the testes causing a testosterone surge. The testosterone surge is called flare phenomenon. Constant exposure to an LHRH agonist causes downregulation of receptors in the pituitary, inhibiting LH and FSH release and decreasing in testosterone production. LHRH agonists should be given with prior administration of antiandrogen of 2 weeks to prevent flare phenomenon.
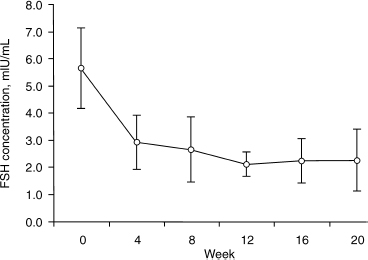
FIGURE 17-3. Effect of Abarelix on serum follicle-stimulating hormone (FSH). (Adapted from Beer TM, Garzotto M, Eilers KM, Lemmon D. Phase II study of abarelix depot for androgen independent prostate cancer progression during gonadotropin-releasing hormone agonist therapy. J Urol 2003;169:1738–1741, with permission.)
Luteinizing Hormone–Releasing Hormone (LHRH) Antagonists
Abarelix, a direct LHRH antagonist, unlike LHRH agonists, avoids the flare phenomenon. A recent phase III randomized trial comparing Abarelix with leuprolide has shown that Abarelix caused rapid medical castration in 24% of men 1 day after treatment and 78% after 7 days compared with 0% of men treated with leuprolide acetate on either day.98 Fig. 17–3 shows the effect of Abarelix on FSH concentrations. A comparable percentage of men achieved and maintained castration between days 29 and 85 in each group. The PSA had a statistically significant decrease for the first month in patients treated with Abarelix. Dihydrotestosterone, LH, PSA, and FSH showed similar rapid reductions without an initial increase.98 As this study does not have a mature follow-up, it is not possible to determine if Abarelix and leuprolide will provide identical rates of disease control.99
Complete Androgen Blockade (CAB)
Ninety percent of serum testosterone is produced by the testis and 10% by the peripheral conversion of the adrenal androgens including dehydroepiandrosterone, dehydroepiandrosterone sulfate, and androstenedione. Testosterone in the prostatic cells is converted to dihydrotestosterone (DHT) by 5α-reductase enzyme. DHT binds with the androgen receptor, which activates the androgen-responsive gene promoting transcription. Labrie et al100 demonstrated that ∼60% of total intraprostatic DHT originates from the testis, whereas 40% is of adrenal origin. Based on this information CAB using LHRH-agonist or bilateral orchiectomy with antiandrogen became a popular treatment of advanced prostate cancer. There have been many studies on whether patients should be treated with CAB or monotherapy alone, creating a great deal of controversy. The Overview Consensus Statement recommends that complete androgen blockade cannot be considered standard therapy in asymptomatic patients. Meta-analyses of clinical trial data would suggest that perhaps 2% to 3% of patients realize a survival benefit with CAB, compared with LHRH agonist alone. It is not possible to identify a subset of patients who will benefit, so combined androgen blockade should be considered the exception rather than the rule.101
Time to Initiate Androgen Deprivation Therapy
When to initiate androgen deprivation therapy (ADT) is another controversial subject in the management of prostate cancer. Based on the results of the 1970s Veterans Administration Cooperative Urological Research Group (VACURG) study102 in which ADT was administered to patients only after the onset of symptoms, the rate of prostate cancer-specific mortality in patients with locally advanced or metastatic disease did not increase. But reanalysis of the VACURG studies and the results of the Medical Research Council Trial103 would suggest that there is benefit to initiating therapy early in terms of disease-specific and overall survival. There is a tendency to treat patients with recurrent prostate cancer with ADT at some point when the PSA is rising prior to symptom development.
Continuous or Intermittent Androgen Deprivation Therapy
Continuous androgen deprivation therapy has been the standard treatment for advanced or metastatic prostate cancer. Intermittent therapy consists of starting ADT and stopping treatment when a predefined clinical response is achieved to let the tumor cells repopulate with androgen-sensitive cells and delay evolution of androgen-insensitive cells. In vitro and animal models of prostate cancer studies suggest that intermittent ADT delays progression to the androgen independent state but does not prevent it.104 This observation has not been confirmed in human clinical trials. The Overview Consensus Statement suggested that given the lack of sufficient long-term data to demonstrate equivalence to conventional ADT, it is mandatory to explain to the patient that intermittent ADT is an investigational rather than a standard approach to ADT, and to document that the patient understands this distinction.101 Goldenberg et al,105 in a very heterogeneous group of prostate cancer patients treated with various forms of intermittent combinations of androgen blockage, reported that during the off-treatment period patients had an improved sense of well-being and near-normal sexual function.
Adverse Effects of Androgen Deprivation Therapy
Androgen deprivation therapy, either bilateral orchiectomy or LHRH-agonist injection, results in a 90% reduction of serum testosterone levels, inducing acute and relatively complete hypogonadism. Clinical symptoms of hypogonadism include loss of libido, poor sexual function, hot flashes, irritability, poor concentration, depression, mood change, reduced cognitive function, weight gain, change of body composition with an increase of fat mass and a decrease of muscle mass, easy fatigability, and loss of bone mineral density leading to osteopenia or osteoporosis and anemia.106–109
Chemotherapy and Prostate Cancer
Despite adequate ADT for advanced prostate cancer, hormone-refractory prostate cancer (HRPC) develops after a median time of 2 years.110 Clinical treatment of HRPC is limited and the patient’s median survival is short, 10 to 12 months.111 Single cytotoxic chemotherapeutic agents including doxorubicin, mitoxantrone, cyclophosphamide, vinblastine, paclitaxel, docetaxel, estramustine, and etoposide have ∼15 to 20% response. Several combinations of these agents have shown significant palliative benefit and antitumor effects in patients with HRPC. Chemotherapy with mitoxantrone and prednisone provides palliation for some patients with symptomatic hormone-resistant prostate cancer.112
Discussion of Case History
In the case at the beginning of this chapter, prostate cancer was diagnosed by early detection rather than by screening. This is a significant cancer, as both the PSA and DRE were abnormal. A healthy 63-year-old man has a life expectancy of 17.06 years.113 Treatment with curative intent is appropriate. With the parameters of a PSA of 5.5 ng/mL, a Gleason score of 3 + 3 = 6, and clinical stage T2a, this patient’s prostate cancer is in a low-risk category. The predicted pathological finding would be organ-confinement 51%, capsule penetration 44%, seminal vesicle involvement 3%, and lymph node involvement 2%.35 This prediction is not 100% proven due to a possible sample error in the biopsy and an inaccurate DRE in determining tumor stage. A Gleason score of 5 to 6 on biopsy corresponded to the same grade in the radical prostatectomy specimen in 64% of the cases.114 The patient does not require a bone scan. The chance of bone metastasis in patients with a PSA less than 10ng/mL and Gleason score less than 7 is less than 1%.115 The treatment options include radical prostatectomy, EBRT, brachytherapy, and watchful waiting. Watchful waiting is not appropriate for this patient for two reasons. First, he has more than 15 years of life expectancy. Though low-risk prostate cancer progresses slowly, if not treated it will eventually pose a threat to his life. Second, studies have shown that clinically localized prostate cancer treated with radical prostatectomy has a cancer-specific mortality of 4.6%, local progression rate of 19.3%, and metastasis of 13.4% versus 8.9%, 61%, and 27.3%, respectively, if treated with watchful waiting.39 There is no prospective randomized control study of sufficient power comparing the efficacy of various local therapies for clinically localized prostate cancer. According to D’amico et al,116 in low-risk prostate cancer the PSA outcome after RP, EBRT, or brachytherapy showed no difference at 5 years. What about a 10-year or 15-year result? A survey showed that the specialists overwhelmingly recommend the therapy they themselves deliver.59
Radical prostatectomy is a good choice for this patient. He has a 90% chance of 15-year disease-specific survival and 74% chance of PSA-progression free in 15 years.41 NHT is not necessary in view of the low PSA, Gleason score, and clinical stage disease. Surgical mortality approaches zero.40 No pelvic lymphadenectomy preceding prostatectomy is necessary, because potential pelvic lymph node involvement is ∼2%.35 Postoperatively, he will have a period of urinary incontinence. Urinary continence gradually improves with Kegel’s exercise with a potential of 3.6% total incontinence.50 If bilateral-nerve sparing is successful, his chance of potency preservation is ∼32%.52 Rectal injury during surgery may occur in 0 to 5.3%47 and bladder contracture in ∼1.3 to 27%.117 Pathological stage and grade of his disease will be available from the prostatectomy specimen. This information is essential to prognosticate his future outcome and therapy. This patient should also see a radiation oncologist for consultation as a potential candidate for radiation therapy. Two radiation modalities are available: EBRT and brachytherapy. Conventional EBRT will provide 75% ten-year disease-free survival61 and 50% biochemically disease free at 10 years.62 If this low-risk patient is treated with 3D-CRT, the 5-year PSA relapse-free survival rate could be 85%.63 Treatment-related toxicity includes bowel complications such as chronic diarrhea, proctitis, rectal or anal stricture, rectal bleeding, or ulcer and urinary tract complications such as cystitis, hematuria, urethral stricture, or bladder contracture.66
Erectile dysfunction occurs in half of patients at 7 years after irradiation.70 Neither NHT nor AHT is necessary for this patient because his disease belongs to a low-risk category. This patient is also a good candidate for brachytherapy, which is probably the least invasive therapy. He will not require NHT because the prostate gland is less than 60 cc. The 10-year PSA progression-free result ranges from 66% to 87%.78,80 The potential side effects of treatment include acute urinary retention (1–14%), incontinence (5–6%), cystitis/urethritis (14%), urethral stricture (1%), proctitis (1–14%), and impotence (4–50%).81 By choosing radiation therapy there is no pathological information available to confirm whether the stage and the grade of the cancer are what is predicted. The patient, armed with all the information, has to balance the benefits and risks of each treatment and make the final choice of therapy. It is highly recommended that he attends the local prostate cancer support group where he can meet with other patients who have undergone the treatment.
Summary and Key Points
• Prostate cancer is the most common cancer and the second leading cause of cancer death of North American men. However, most men die with prostate cancer rather than die of prostate cancer.
• There is a great deal of variation in the international incidence of prostate cancer. The highest incidence is in Jamaicans, and the second highest is in African Americans; the lowest incidence is in the Chinese who live in China.
• The unmodifiable risk factors of prostate cancer include age and genetics, and the modifiable risk factors include diet and lifestyle.
• There is heated debate about whether men should be screened for prostate cancer. There is no evidence that screening and early treatment of prostate cancer will reduce mortality of the disease. On the other hand, there is no evidence that it will not.
• It is reasonable to offer annual PSA and DRE to men 50 years or older who a have life expectancy of greater than 10 years. Men at high risk, such as African Americans and men who have a first-degree relative diagnosed with prostate cancer at an early age, should begin testing at age 45.
• Information about the potential benefits and harms of screening and treatment of prostate cancer and the limits of current evidence of screening and treatment of prostate cancer should be thoroughly discussed with patients before testing.
• Patients diagnosed with clinically localized prostate cancer face a difficult task of choosing a treatment option.
• Patients should take active participation in decision making with regard to choices of treatment modality after full consultation with various specialists.
• Future research should aim at cancer prevention and discovering factors that can help identify significant prostate cancer that requires treatment and insignificant prostate cancer that will remain occult the rest of the patient’s lifetime and requires no treatment.
• For prostate cancer that has progressed beyond curability, a method should be available to convert the disease to a controllable chronic illness.
REFERENCES
1. American Cancer Society. Cancer Facts and Figures 2003, using 2000 population standard for age adjustment.
2. Carter HB, Piantadosi S, Isaacs JT. Clinical evidence for and implications of the multistep development of prostate cancer. J Urol 1990;143:742–746
3. Glover FE Jr, Coffey DS, Douglas LL, et al. The epidemiology of prostate cancer in Jamaica. J Urol 1998;159:1984–1986
4. Ruijter E, van de Kaa C, Miller G, et al. Molecular genetics and epidemiology of prostate carcinoma. Endocr Rev 1999; 20:22–45
5. Miller G. Prostate cancer among the Chinese: pathologic, epidemiologic, and nutritional considerations. In: Resnick MI, Thomspon IM, eds. Advanced Therapy of Prostate Disease. New York: BC Decker; 2000:18–27
6. Wingo PA, Tong T, Bolden S. Cancer statistics, 1995. CA Cancer J Clin 1995;45:8–31
7. Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst 1996;88:1118–1126
8. Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349:215–224
9. Von Eschenbach A, Ho R, Murphy GP, et al. American Cancer Society guideline for the early detection of prostate cancer: update 1997. CA Cancer J Clin 1997;47:261–264
10. Johansson JE, Holmberg L, Johansson S, Bergstrom R, Adami HO. Fifteen-year survival in prostate cancer: a prospective, population-based study in Sweden. JAMA 1997;277:467–471. Erratum in JAMA 1997;278:206
11. Thompson IM, Goodman PJ, Tangen CM. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349:215–224
12. Arcangeli CG, Ornstein DK, Keetch DW, Andriole GL. Prostate-specific antigen as a screening test for prostate cancer. The United States experience. Urol Clin North Am 1997; 24:299–306
13. Fang J, Metter EJ, Landis P, Chan DW, Morrell CH, Carter HB. Low levels of prostate-specific antigen predict long-term risk of prostate cancer: results from the Baltimore Longitudinal Study of Aging. Urology 2001;58:411–416
14. Riffenburgh RH, Amling CL. Use of early PSA velocity to predict eventual abnormal PSA values in men at risk for prostate cancer. Prostate Cancer Prostatic Dis 2003;6:39–44
15. Partin AW, Carter HB. The use of prostate-specific antigen and free/total prostate-specific antigen in the diagnosis of localized prostate cancer. Urol Clin North Am 1996;23:531–540
16. Naitoh J, Zeiner RL, Dekernion JB. Diagnosis and treatment of prostate cancer. Am Fam Physician 1998;57:1531–1539
17. Smith DS, Catalona WJ, Herschman JD. Longitudinal screening for prostate cancer with prostate-specific antigen. JAMA 1996;276:1309–1315
18. Wahid ZU. Task force finds evidence lacking on whether routine screening for prostate cancer improves health outcomes. J Natl Med Assoc 2003;95:A20
19. Lubke WL, Optenberg SA, Thompson IM. Analysis of the first-year cost of a prostate cancer screening and treatment program in the United States. J Natl Cancer Inst 1994;86: 1790–1792
20. Sanchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate 2003;54:238–247
21. American Cancer Society Guidelines for the Early Detection of Cancer, 2002
22. Carroll P, Coley C, Mcleod D, et al. Early detection and diagnosis of prostate cancer. Urology 2001;57:217–224
23. Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventative Services Task Force. Ann Intern Med 2002;137:917–929
24. Ferrini RL, Woolf SH. American College of Preventive Medicine practice policy: screening for prostate cancer in American men. Am J Prev Med 1998;15:81–84
25. Gohagan JK, Prorok PC, Kramer BS, et al. Prostate Cancer screening in the prostate, lung, colorectal and ovarian cancer screening trial of the National Cancer Institute. J Urol 1994; 152:1903–1904
26. Standaert B, Louis Denis L. The European randomized study of screening for prostate cancer: an update. Cancer 1997;80: 1830–1834
27. Basler JW, Thompsen IM. Lest we abandon digital rectal examination as a screening test for prostate cancer. J Natl Cancer Inst 1998;90:1761–1763
28. Bretton PR. Prostate-specific antigen and digital rectal examination in screening for prostate cancer: a community-based study. South Med J 1994;87:720–723
29. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med 1991;324:1156–1161
30. Catalona WJ, Partin AW, Slawin KM, et al. Southwick use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA 1998;279: 1542–1547
31. Ellis WJ, Chetner MP, Preston SD, Brawer MK. Diagnosis of prostate carcinoma: the yield of serum prostate specific antigen, digital rectal examination, and transrectal ultrasonography. J Urol 1994;152:1520–1525
32. Mikolajczyk SD, Marker KM, Millar LS, et al. A truncated precursor form of prostate-specific antigen is a more specific serum marker of prostate cancer. Cancer Res 2001;61: 6958–6963
33. Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology 2001;58:1008–1015
34. Nam RK, Diamandis EP, Toi A, et al. Serum human glandular kallikrein-2 protease levels predict the presence of prostate cancer among men with elevated prostate-specific antigen. J Clin Oncol 2000;18:1036–1042
35. Partin AW, Kattan MW, Subong EN, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer: a multi-institutional update. JAMA 1997;277:1445–1451
36. Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer J Natl Cancer Inst 1998;90:766–771
37. Lattouf JB, Saad F. Gleason score on biopsy: is it reliable for predicting the final grade on pathology? BJU Int 2002;90:694–698
38. Albertsen PC, Hanley JA, Gleason DF, Barry MJ. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA 1998;280:975–980
39. Holmberg L, Bill-Axelson A, Helgesen F, et al. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N Engl J Med 2002;347:781–789
40. Catalona WJ, Carvalhal GF, Mager DE, Smith DS. Potency, continence, and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol 1999;162: 433–438
41. Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy: the 15 year Johns Hopkins experience. Urol Clin North Am 2001;28: 555–565
42. Reiner WG, Walsh PC. An anatomical approach to the surgical management of the dorsal veins and Santorini’s plexus during radical retropubic surgery. J Urol 1979;121:198–200
43. Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol 1982;128: 492–497
44. Stamey TA. Techniques for avoiding positive surgical margins during radical prostatectomy. Atlas of the Urol Clin North Am 1994; 2:37–51
45. Walsh PC. Anatomic Radical Retropubic Prostatectomy; Campbell Urology. 7th ed. Philadelphia: WB Saunders; 1998
46. Davis M, Sofer M, Gomez-Marin O, Bruck D, Soloway MS. The use of cell salvage during radical retropubic prostatectomy: does it influence cancer recurrence? BJU Int 2003;91: 474–476
47. Shekarriz B, Upadhyay J, Wood DP. Intraoperative, perioperative, and long-term complications of radical prostatectomy. Urol Clin North Am 2001;28:639–653
48. Hedican SP, Walsh PC. Postoperative bleeding following radical prostatectomy. J Urol 1994;152:1181–1183
49. Steiner MS, Morton RA, Walsh PC. Impact of anatomical radical prostatectomy on urinary continence. J Urol 1991;145: 512–515
50. Murphy GP, Mettlin C, Menck H, et al. National patterns of prostate cancer treatment by radical prostatectomy: results of a survey by the American College of Surgeons Committee on Cancer. J Urol 1994;152:1817–1819
51. Quinlan DM, Epstein JI, Carter BS, Walsh PC. Sexual function following radical prostatectomy: influence of preservation of neurovascular bundle. J Urol 1991;145:998–1002
52. Geary ES, Dendinger TE, Freiha FS, Stamey TA. Nerve sparing radical prostatectomy: a different view. J Urol 1995;154: 158–159
53. Kim HL, Mhoon DA, Brendler CB. Does the CaverMap device help preserve potency? Curr Urol Rep 2001;2:214–217
54. Kim ED, Scardino PT, Hample O, Mills NL, Wheeler TM, Nah RK. Interposition of sural nerve restore function of cavernous nerves resected during radical prostatectomy. J Urol 1999;161: 188–192
55. Meuleman EJH, Mulders PFA. Erectile function after radical prostatectomy: a review. Eur Urol 2003;43:95–102
56. Zippe CD, Kedia AW, Kedia K, Nelson DR, Agarwal A. Treatment of erectile dysfunction after radical prostatectomy with sildenafil citrate (Viagra). Urology 1998;52:963–966
57. Linet OI, Ogrinc FG. Efficacy and safety of intracavernosal alprostadil in men with erectile dysfunction: the Alprostadil Study Group. N Engl J Med 1996;334:873–877
58. Padma-Aathan H, et al. Treatment of men with erectile dysfunction with transurethral alprostadil. N Engl J Med 1997; 336:1–7
59. Fowler FJ Jr, Collins M, Albertsen PC, Zietman A, Elliott DB, Barry MJ. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA 2000;283:3217–3222
60. Poon M, Ruckle H, Bamshad BR, Tsai C, Webster R, Lui P. Radical retropubic prostatectomy bladder neck preservation versus reconstruction. J Urol 2000;163:194–198
61. Roach M III, Lu J, Pilepich MV, et al. Long-term survival after radiotherapy alone: Radiation Therapy Oncology Group Prostate Cancer Trials. J Urol 1999;161:864–868
62. Zietman AL, Coen JJ, Dallow KC. Shipley WU. The treatment of prostate cancer by conventional radiation therapy: an analysis of long-term outcome. Int J Radiat Oncol Biol Phys 1995;32:287–292
63. Zelefsky MJ, Leibel SA, Gaudin PB, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys 1998;41:491–500
64. Lukka H, Warde P, Pickles T, Morton G, Brundage M, Souhami L. Controversies in prostate cancer radiotherapy: consensus development. Can J Urol 2001;8:1314–1322
65. Zelefsky MJ, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys 2002;53:1111–1116
66. Lawton CA, Won M, Pilepich MV, et al. Long-term sequelae following external beam irradiation for adenocarcinoma of the prostate: analysis of RTOG studies 7506 and 7706. Int J Radiat Oncol Biol Phys 1991;21:935–939
67. Shipley WU, Zeitman AL, Hanks GE, et al. Treatment related sequelae following external beam radiation for prostate cancer: a review with an update of patients with stages T1 and T2 tumor. J Urol 1994;152:1799–1803
68. Leibel SA, Hanks GE, Kramer S. Patterns of care outcome studies: results of the national practice in adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys 1984;10:401–409
69. Smith WG, Helle PA, Putten WL, et al. Late radiation damage in prostate cancer patients treated by high dose external radiotherapy in relation to rectal dose. Int J Radiat Oncol Biol Phys 1990;18:23–29
70. Asbell SO, Krall JM, Pilepich MV, et al. Elective pelvic irradiation in stage A2, B carcinoma of the prostate: analysis of RTOG 77–06. Int J Radiat Oncol Biol Phys 1988;15: 1307–1316
71. Whitmore WF Jr, Hilaris B, Grabstald H. Retropubic implantation of iodine-125 in the treatment of prostate cancer. J Urol 1972;108:918–920
72. Schellhammer PF, L-el Mahdi AE, Ladaga LE, Schutheiss T. Iodine implantation for carcinoma of the prostate: 5-year survival free of disease and incidence of local failure. J Urol 1985;134:1140–1145
73. Zelefsky MJ, Whitmore WF Jr. Long-term results of retropubic permanent 125-iodine implantation of the prostate for clinically localized prostatic cancer. J Urol 1997;158: 23–29
74. Holm HH, Juul N, Pedersen JF, Hansen H, Stroyer I. Transperineal iodine seed implantation in prostatic cancer guided by transrectal ultrasonography. J Urol 1983;130:283–286
75. Blasko JC, Ragde H, Grimm PD. Transperineal ultrasound guided implantation of the prostate: morbidity and complications. Scand J Urol Nephrol Suppl 1991;137:113–118
76. Ellis WJ. Prostate brachytherapy Cancer Metastasis Rev 2002; 21:125–129
77. Blasko JC, Mate T, Sylvester JE, Grimm PD, Cavanagh W. Brachytherapy for carcinoma of the prostate: techniques, patient selection, and clinical outcomes. Semin Radiat Oncol 2002;12:81–94
78. Nag S, Beyer D, Friedland J, Grimm P, Nath R. American brachytherapy society (ABS) recommendations for transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys 1999;44:789–799
79. Benoit RM, Cohen JK, Miller RJ Jr, Merlotti L. Role of Cryosurgery in the Treatment of Prostate Cancer: Advanced Therapy of Prostate Disease. London: BC Decker Hamilton; 2000
80. Ragde H, Korb LJ, Elgamal A, Grado GL, Nadir BS. Modern prostate brachytherapy prostate specific antigen results in 219 patients with up to 12 years of observed follow-up. Cancer 2000;89:135–141
81. Crook J, Lukka H, Klotz L, et al. Systematic overview of the evidence for brachytherapy in clinically localized prostate cancer. CMAJ 2001;164:975–981
82. Long JP, Bahn D, Lee F, Shinnohara K, Chinn DO, Macaluso JN Jr. Five-year retrospective, multi-institutional pooled analysis of cancer-related outcomes after cryosurgical ablation of the prostate. Urology 2001;57:518–523
83. Long JP. New methods of focal ablation of the prostate. In: Kantoff PW, Carroll PR, D’Amico AV. Prostate Cancer, Principles and Practice. Philadelphia: Lippincott Williams & Wilkins; 2002;358–367
84. Uchida T, Sanchvi NT, Gardner TA, et al. Transrectal high-intensity focused ultrasound for treatment of patients with stage T1b-2N0M0 localized prostate cancer: a preliminary report. Urology 2002;59:394–399
85. Huggins C, Hodges CV. Studies on prostate cancer, I: the effect of castration, of estrogen, and of androgen injection on serum phosphatase in metastatic carcinoma of the prostate. Cancer Res 1941;1:293
86. Gleave ME, La Bianca S, Goldenberg SL. Review of neoadjuvant hormonal therapy prior to radical prostatectomy: promises and pitfalls. Prostate Cancer Prostatic Dis 2000;3: 136–144
87. Pilepich MV, Krall JM, Al-Sarraf M, et al. Androgen deprivation with radiation therapy compared with radiation therapy alone for locally advanced prostatic carcinoma: a randomized comparative trial of the Radiation Therapy Oncology Group. Urology 1995;45:616–623
88. Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med 1999;341:1781–1788
89. Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 1997;337:295–300
90. Schulman CC, Altwein JE, Zlotta AR. Treatment options after failure of local curative treatments in prostate cancer: a controversial issue. BJU Int 2000;86:1014–1022
91. Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999;281:1591–1597
92. Critz FA, Levinson AK, Williams WH, Holladay DA, Holladay CT. The PSA nadir that indicates potential cure after radiotherapy for prostate cancer. Urology 1997;49:322–326
93. Pisters LL, von Eschenbach AC, Scott SM, et al. The efficacy and complications of salvage cryotherapy of the prostate. J Urol 1997;157:921–925
94. Chon JK, Jacobs SC, Naslund MJ. The cost value of medical versus surgical hormonal therapy for metastatic prostate cancer. J Urol 2000;164:735–737
95. Cox LE, Crawford ED. Estrogens in the treatment of prostate cancer. J Urol 1995;154:1991–1998
96. Robertson CN, Roberson KM, Padilla GM, et al. Induction of apoptosis by diethylstilbestrol in hormone-insensitive prostate cancer cells. J Natl Cancer Inst 1996;88:908–917
97. Iversen P, Tyrrell CJ, Kalsary AV, et al. Bicalutamide monotherapy compared with castration in patients with non-metastatic locally advanced prostate cancer: 6.3 years of follow-up. J Urol 2000;164:1579–1582
98. McLeod D, Zinner N, Tomera K, et al. A phase 3, multicenter, open-label, randomized study of abarelix versus leuprolide acetate in men with prostate cancer. Urology 2001;58:756–761
99. Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin 2002;52: 154–179
100. Labrie F, Dupont A, Belanger A. Complete androgen blockade for the treatment of prostate cancer. In: DeVica VP Jr, Hellman S, Rosenberg SA, eds. Important Advances in Oncology. Philadelphia: JB Lippincott; 1985:193–271
101. Carroll PR, Fair WR, Grossfeld GD, et al. Overview consensus statement. Urology 2001;58:1–4
102. Byar DP. Proceedings: the Veterans Administration Cooperative Urological Research Group’s studies of cancer of the prostate. Cancer 1973;32:1126–1130
103. The Medical Council Prostate Cancer Working Party Investigators Group. Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. Br J Urol 1997;79:235–246
104. Akakkura K, Bruchovsky N, Goldenberg SL, et al. Effects of intermittent androgen suppression on androgen-dependent tumors. Cancer 1993;71:2782–2789
105. Goldenberg SL, Bruchovsky N, Gleave ME, et al. Intermittent androgen suppression in the treatment of prostate cancer: a preliminary report. Urology 1995;45:839–845
106. Stone P, Hardy J, Huddart R, et al. Fatigue in patents with prostate cancer receiving hormone therapy. Eur J Cancer 2000;36:1134–1141
107. Tayek JA, Heber D, Byerley LO, et al. Nutritional and metabolic effects of gonadotropin-releasing hormone agonist treatment for prostate cancer. Metabolism 1990;39: 1314–1319
108. Diamond T, Campbell J, Bryant C, et al. The effect of combined androgen blockage on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer 1998;83:1561–1566
109. Atala A, Amin M, Garty JI. Diethylstilbestrol in treatment of post-orchiectomy vasomotor symptoms and its relationship with serum follicle-stimulating hormone, luteinizing hormone, and testosterone. Urology 1992;39:108–110
110. Scher HI, Steineck G, Kelly WK. Hormone-refractory prostate cancer: refining the concept. Urology 1995;46:142–148
111. Mahler C, Denis CJ. Hormone-refractory disease. Semin Surg Oncol 1995;11:77–83
112. Tannock IE, Osolba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 1996; 14:1756–1764
113. Period Life Table. Updated February 2003, Social Security Administration (www.ssa.gov/)
114. Steinberg DM, Sauvageot J, Piantadosi S, et al. Correlation of prostate needle biopsy and radical prostatectomy Gleason grade in academic and community settings. Am J Surg Pathol 1997;21:566–576
115. Gleave ME, Coupland D, Drachenberg D, Cohen L, Kwong S, Goldenberg SL. Ability of serum prostate-specific antigen levels to predict normal bone scans in patients with newly diagnosed prostate cancer. Urology 1996;47:708–712
116. D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969–974
117. Surya BV, Provet J, Johanson KE, et al. Anastomotic strictures following radical prostatectomy: risk factors and management. J Urol 1990;143:755–758
< div class='tao-gold-member'>