19
Bioidentical Hormone Replacement with Testosterone in Men
Case History
Mr. J.J. is a 49-year-old man who has been referred for management of hypogonadism. Another physician told him that his levels of total testosterone were below 300 ng/dL. His wife, who is convinced that his symptoms were attributed to his low testosterone state, accompanied him to the appointment. On direct questioning, he admitted to tiredness, loss of libido, mood changes, and memory loss. He admitted to high stress in his job, as his business was failing. He was not particularly happy to be in the doctor’s office, but said that his wife had insisted that something be done about him. He gave a past medical history of coronary artery disease, hypercholesterolemia, and a transient ischemic attack (TIA). He drinks up to 24 alcoholic beverages a week. His current medications included Cozaar, Lipitor, and aspirin. Physical examination revealed a facial blush and rhinophyma suggestive of alcoholic abuse. The liver was not enlarged, but he had an increased waist-hip ratio. Blood pressure (BP) was 136/78 mmHg, weight was 178 lbs, and body fat content by caliper was 26.5%. A repeat morning sample of total testosterone revealed a level of 267 ng/dL. His prolactin, ferritin, estradiol, luteinizing hormone (LH), and prostate-specific antigen (PSA) were in the normal range.
His wife said that they wanted to explore various options for testosterone replacement therapy and mentioned that cost may be an issue because they have only calamity insurance. It was stressed to the patient that chronic alcoholism certainly can suppress testosterone levels and that he needed to stop drinking completely. Various options were discussed with them, and they felt they wanted to try a commercial topical version of 50 mg testosterone (Androgel) priced at approximately $120 per month. After a few weeks, the dose was escalated to 100 mg but the patient had felt not much change in his mood or memory, although he did acknowledge that his libido was improved. At this point, the wife asked if he could try compounded testosterone ($50 per month), and a similar dose of the generic gel testosterone was prescribed. Laboratory testing revealed poor absorption of this particular version of compounded testosterone. It was explained to the patient that it could have been due to skin absorption characteristics, as a small minority of patients do not absorb well. The patient said that the pharmacist had told him that an injection might work better. The patient obtained a 10-dose, 200 mg each dose, vial for $89.95, not including the cost of the needles and syringes. His wife, who was an ex-nurse, was comfortable with injecting him every 2 weeks. After a year of injections, the patient said that he did not find any major improvements in symptoms as compared with the gels, and at most had a slight improvement in symptoms in only the first week of the injection. At this point, he wanted to consider a testosterone implant, and was asked to return to the clinic for further counseling.
History of Testosterone Replacement in Men
The association of the testicles with sexual and reproductive function dates back to thousands of years B.C., with accounts of the Babylonians castrating men as punishment for crimes such as adultery, and even castrating young choir boys in the early Christian Church to preserve their soprano voices. Animal husbandry depended on the ability to preserve the potency of desired studs, while removing other males from the gene pool and domesticating them via castration.
The premodern approach to androgen enhancements consisted of a variety of untested, possibly harmful, and dubiously effective concoctions that doctors and charlatans alike devised and sold to the public without scientific testing. These included oral preparations of the testicles of various animals that purportedly enhanced virility and sexual potency. The famous French physician Brown-Sequard may have inadvertently started the field of reproductive endocrinology in 1889, when he began performing uncontrolled experiments whereby he injected dogs (and even himself) with aqueous extracts of animal testes. Brown-Sequard published exuberant accounts of the improvement in their general health, muscular strength, and even cognitive functions. These and other reports led to the fad of using the testicles of every animal available, including monkeys, bulls, and pigs, to prepare both aqueous and glycerol extracts that were then injected or ingested into well-to-do patients who believed they were onto a veritable fountain of youth. This came to an abrupt, and appropriate, halt when an international committee of pragmatic physicians made a proclamation that these claims of rejuvenation were unfounded and probably unsafe.
Thus, androgen research was thrown back into the hands of the appropriate people: scientists. By the end of the 1920s, several researchers developed bioassays of androgen potency using the regeneration of sex organs of castrated mice and rats, or, similarly, the growth of the rooster comb and waddle in capons. Combined with the advancing techniques of modern organic chemistry, these researchers eventually isolated various “male hormones” from the urine of human and animal males and laid the groundwork for the eventual identification of testosterone.
In May 1935, a chemist in Amsterdam named David, working with the famous group of Laqueur and Freud, isolated 10 mg of a pure crystalline compound from 100 kg of bull testes. He named it “testosterone.” It turned out to be identical to a compound that had been synthesized by Butenandt and Ruzicka from cholesterol. Thus, not only did scientists now have the most active male hormone in a purified state, they knew its chemical structure and how to synthesize it from cholesterol. Within a few months Ruzicka and his group reported the synthesis of an orally active form of testosterone made by substituting a methyl group at the 17 position of the original molecule. It was called methyltestosterone.
So as early as 1935, scientists were studying an oral form of testosterone and at the same time synthesizing hundreds of new compounds based on the original steroid nucleus of the testosterone molecule. Because it is a three-dimensional molecule, the number of stereoisomers, α and β positions of the side groups, and the potential to add various other side groups and oxidize or reduce the A ring are important factors, there are thousands of possible cogeners that could be produced for experimentation. Fortunately, for the purpose of our discussion we need only mention a handful of them.
Oral Androgens
Characteristics of Oral Agents for Androgen Replacement
The perfect agent for androgen replacement therapy should have the following characteristics:
1. Deliver androgen in a physiological manner
2. Have a reproducible and predictable pharmacological profile
3. Be amenable to routine laboratory assay so that levels can be monitored
4. Completely reverse the signs and symptoms of androgen deficiency
5. Be unattractive to those who would abuse such compounds
6. Be affordable
7. Be easily administered in a patient-friendly manner
It is helpful to look at the classification of testosterone modifications (Figs. 19–1 and 19–2 [pp. 171, 172, respectively]). As research progressed with both the oral and injectable forms of testosterone, scientists became aware of the two somewhat distinct actions of the various androgens they were using. Anabolic effects were those features that caused a positive nitrogen balance and increased the mass of various tissues and organs such as muscle, bone, and blood. Androgenic or virilizing effects were those features that caused the development of facial and body hair, increased sebum production (and acne), deepening of the voice, and enlargement of the prostate gland and other male reproductive organs.
Researchers throughout the 1940s, 1950s, and 1960s used various animal biological assays to assess the anabolic versus the androgenic actions of their newly developed compounds. Hypertrophy of the castrated rat kidney or levator ani muscle was used as an indicator of anabolic activity, whereas the same effect on the rodent prostate or seminal vesicles was used to assess the androgenic properties of a newly developed steroid. The search for a pure anabolic agent without virilizing effects was finally found to be futile, as all of the tested molecules would eventually have virilizing effects in humans. This was particularly unacceptable in women and children for whom agents for breast cancer or aplastic anemia were being sought.
What those researchers did not know is that there is a single androgen receptor in humans, and that unlike the estrogen receptor, which has α and β forms that are tissue specific, they would not be successful at separating the effects on individual tissues.
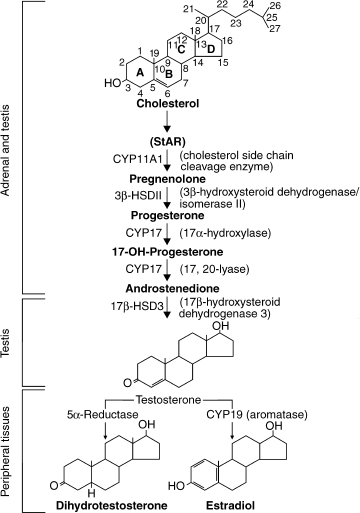
FIGURE 19–1. Pathway of testosterone formation in the testis and the conversion of testosterone to active metabolites in peripheral tissues (sTAR, steroidogenic acute regulatory protein). (Adapted from Griffin JE, Wilson JD. Disorders of the testes. In: Braunwald E, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 15th ed. New York: McGraw-Hill; 2001, with permission.)
Interestingly, we now can explain the effects of the various synthetic agents on humans by identifying how these molecules compare with the endogenous natural steroids that are metabolized in vivo. It is now apparent that the net effects of testosterone (T) in the human male are the sum of the effects of the two major metabolites of T [E2 and dihydrotestosterone (DHT)] as well as the effects of T itself. Many tissues, such as adipose tissue, have high concentrations of an aromatase enzyme that converts T into estradiol (E2). Other tissues such as the skin (especially in the perineal region and scrotum) have high concentrations of a 5α–reductase enzyme that converts T into 5α-dihydrotestosterone (DHT). E2 is responsible for many important effects and side effects of T therapy including raising the high-density lipoprotein (HDL), closure of bone epiphyses, and the complete formation of strong bones. DHT, while binding the same receptor as T, is responsible for the development and hypertrophy of the prostate and also for many of the skin effects that we see from T therapy.
Certain modifications of the A ring of the steroid nucleus makes the molecule behave like DHT. DHT, which we now know binds five to 10 times more tightly to the androgen receptor than does T. DHT is also nonaromatizable, thus it cannot be turned into E2, and therefore will not have the benefits of an estrogen (such as raising HDL cholesterol or closing bone epiphyses), nor will it have the undesirable side effects such as causing gynecomastia. In contrast, the esterified cogeners of T are slowly released into the circulation and then serum esterases hydrolyze the fatty acid off the 17 position and convert it into pure testosterone, thus producing a molecule that is capable of being 5α reduced to form DHT, or aromatized at the A ring to produce E2.
The Physicians’ Desk Reference (2003 edition) lists only three orally active anabolic-androgenic steroids for current use. These three, methyltestosterone (methyl-T), oxandrolone, and oxymethenolone, all have specific Food and Drug Administration (FDA) indications, even though their differences are more quantitative than qualitative. Oxandrolone is indicated as an adjunctive therapy to promote weight gain following extensive surgery, chronic infections (AIDS), or severe trauma. It is given orally as a 2.5 -mg tablet, one to two tablets up to four times a day. Its use is not restricted to males, but obvious limitations and precautions must be observed in females. Oxymethenolone (Anadrol-50) is only FDA approved for the adjunctive treatment of aplastic anemias. It is dosed in children and adult at 1 to 5 mg/kg/day. It comes as a 50-mg tablet. Methyl-testosterone is available as a 10-mg tablet by itself (Testred) or in two different dosage combinations with esterified estrogens (in years past, it was also available as a 10-mg orally dissolving tablet that absorbed directly through the venous and lymphatic beds under the tongue). Testred is the only oral FDA approved agent for male hypogonadism, whereas the latter two are used for hormone replacement therapy (HRT) in postmenopausal women in situations where the treating physician believes the T will aid in libido, bone density, or muscle acquisition. For the reasons that will be listed later, methyl-T is seldom used for male HRT, but when it is, a dose of ∼50-mg/day, divided q.i.d., is required. As an addition to female HRT, methyl-T has enjoyed some definite, but limited, popularity. It is felt by most experts that the low doses used by females are not a health threat or likely to cause liver dysfunction.
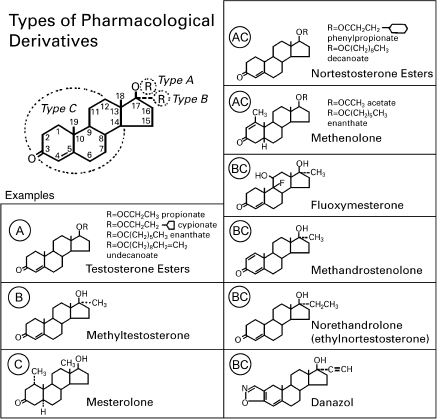
FIGURE 19–2. Types of androgen preparations available for clinical use. Type A derivatives are esterified in the 17(3 position. Type B derivatives have alkyl substitutions in the 17α position. Type C derivatives involve a variety of alterations of ring structure that enhance activity, impede catabolism, or influence both functions. Most androgen preparations involve combinations of type AC or type BC changes.
The oral route of administration for these agents exposes the liver to very high doses. This has been associated with the rare, but concerning, development of two liver problems. The most common is cholestatic jaundice. This reversible event is generally not accompanied by active liver damage or significant rise in the transaminases. It is considered to be completely reversible upon cessation of the oral steroid. In contrast to this, the long-term use of high doses of some of these agents has led to the much more serious development of peliosis hepatis. This formerly rare disorder causes the formation of blood-filled tumors in the liver that can hemorrhage or otherwise lead to serious liver damage. Friedl,1 in a review of this subject, found 90 cases of reported peliosis in oral and injected steroid users. Other series also found an alarming incidence of this condition in oral steroid users being treated with high doses for aplastic anemia (seven of 19 versus one of 28 in patients not treated with oral steroids). It has also been linked to the development of hepatomas and adenomas of the liver. In his review, Friedl noted 91 reported cases as of 1991 in oral steroid users. Many of these events occurred in patients with Fanconi’s syndrome. Some of them actually seemed to regress after withdrawal of the androgenic steroids.
So how do the oral steroid agents hold up to the seven requirements mentioned at the beginning of this chapter for the ideal agent for the treatment of hypogonadism? Well, (1) they don’t represent a physiological delivery system; (2) their results are not always reproducible in heterogeneous populations; (3) we cannot easily measure their levels in the bloodstream; (4) because they are not aromatizable they do not reverse all of the signs and symptoms of hypogonadism; (5) they are extensively abused; (6) despite their having been around for more that 50 years they are still extremely expensive (>$600/month for some); (7) and because of multiple doses required per day, they are not convenient. Thus, they satisfy none of the seven requirements, and they have the potential to cause great harm. It is one of the great ironies of medicine, that after 60 years of research by countless scientists, the preferred agent for the reversal of hypogonadism is still testosterone itself.
TESTOSTERONE UNDECANOATE
The undecanoate form of testosterone, marketed as Andriol in Europe and Asia, has yet to be approved by the FDA at the time of this writing. This form of oral testosterone is safer than those mentioned previously, but requires dosing three times daily. Surprisingly, testosterone undecanoate is the most widely used androgen worldwide, but still not available in the United States. Testosterone undecanoate is an oral androgen that provides the hypogonadal patient with the unmodified testosterone molecule. It was introduced in the mid-1970s. In a study by Gooren over 10 years, a small sample of men had biochemical parameters of liver function followed, and the levels remained constant during the study period, indicating that there is no increased hepatic enzymatic breakdown of the androgen over time. Over the 10-year period, some patients on testosterone undecanoate developed mild obstruction of urine flow. Digital examination of the prostate did not reveal signs of prostate tumors.2 Testosterone undecanoate appears to be a safe oral androgen, but experience in the United States is lacking.
Injectable Androgens
Intramuscular injections of testosterone, usually as an enanthate or cypionate ester, do not have to be given daily, but are instead given every 1–2 weeks. After injections, blood levels peak about 2 to 3 days after dosing and slowly decline during the next 1 to 2 weeks. The injections are mildly painful, and rise and fall in serum levels of testosterone over time may be accompanied by changes in mood and the sense of well-being. This is called the “roller-coaster” effect, whereby the patient feels best for a week or so after the injection, then loses this effect over the next several days. Arguably, it may also cause a higher rise in PSA as compared with more physiological preparations of testosterone. Injectable therapy usually is the least expensive way to provide testosterone replacement, and it requires the least patient motivation and compliance. Sih and his colleagues3 validated this form of testosterone replacement. Their study was undertaken to examine the year-long effects of intramuscular testosterone administration in older men. In that study, 15 hypogonadal men (mean age 68 ± 6 year) were randomly assigned to receive a placebo, and 17 hypogonadal men (mean age 65 ± 7 year) were randomly assigned to receive testosterone. Hypogonadism was defined as a bioavailable testosterone <60ng/dL. The men received injections of placebo or 200 mg testosterone cypionate biweekly for 12 months. The main outcomes measured included grip strength, hemoglobin, PSA, leptin, and memory. The men in the testosterone had greater improvement in bilateral grip strength and decreases in leptin than did those assigned to the control group. There were no significant changes in PSA or memory. Several study subjects were withdrawn because of an abnormal elevation in hematocrit. Intramuscular testosterone supplementation improved strength, increased hemoglobin, and lowered leptin levels in older hypogonadal men. Intramuscular testosterone may have a role in the treatment of frailty in men with hypogonadism and perhaps in controlling obesity through its effects on leptin.2
Injectable Testosterone Undecanoate
This form of injectable testosterone is yet to be available in the United States. However, German investigators studied the suitability of intramuscular testosterone undecanoate injections for substitution therapy in hypogonadal men,4 and it has also been studied for birth control.
The study was small in size and descriptive, and investigators found that testosterone serum levels were never below the lower limit of normal and only briefly after the third and fourth injection above the upper limit of normal, whereas peak and trough values increased over the 24-week observation period. Estradiol and dihydrotestosterone followed this pattern, not exceeding the normal limits. No serious side effects were noted in this small study. Slight increases in body weight, hemoglobin, hematocrit, prostate volume, and PSA, and suppression of gonadotrophins as well as increased ejaculation frequency occurred as signs of adequate testosterone substitution. In the future, injectable testosterone undecanoate may be well suited for long-term substitution therapy in hypogonadism and perhaps even hormonal male contraception.
Injectable Nandrolone
This form of androgen substitution is commonly abused by athletes because of its effects on skeletal muscle and strength. Its effects on sexuality are not pronounced. Medical therapeutic indications for nandrolone in some circumstances may include the HIV wasting syndrome and end-stage renal failure. An Australian study determined the efficacy of nandrolone in HIV wasting syndrome.5 The changes in weight and body composition (lean body mass, total body water, and nitrogen index) were measured by anthropometry, bioelectrical impedance, and in vivo neutron activation. Subjects who failed to gain weight (10.9%) were treated with nandrolone decanoate (100mg/mL) by deep intramuscular injection every 2 weeks for 16 weeks. Changes in quality of life were assessed by a short questionnaire. Changes in biochemistry, hematology, and immunology were also measured. The investigators found significant increases in weight (mean, 0.14kg per week; p<.05) and lean body mass (mean, 3 kg by anthropometry; p < .05). The change in lean body mass was of similar magnitude across all measurement modalities. Quality of life parameters, especially functionality, increased significantly during the trial. No subject experienced toxicity.
Sublingual Testosterone
A few published reports have defined the characteristics and pharmacokinetics of sublingual T. This form of androgen replacement has several advantages, but ultimately is unsuitable for chronic replacement therapy because of its large peak and trough levels and the very short half-life.
T cyclodextrin consists of unadulterated T passively carried inside a sphere of α1–4 linked glucopyranose molecules. These oligosaccharide macro rings are quite hydrophilic on their exterior, and the T molecule is held in the relatively apolar center. When placed into the mouth, the lipophilic T passively traverses the buccal mucosa and rapidly enters the bloodstream. The remaining carbohydrate moiety is passed into the digestive tract and broken down into harmless, inactive metabolites.
In the late 1990s, the research team at Harbor-UCLA published three excellent studies of a sublingual product that was being investigated and developed by Biotechnology General Corporation. Both 2.5- and 5.0-mg sublingual tablets were compared with T enanthate injectable depot.6 The results showed that peak serum values were reached in 20 minutes and were near baseline by 2 or 3 hours. Peak levels with the 2.5-mg dose ranged from 800 to 1100ng/dL. The peak levels of the 5.0-mg dose went as high as 1500ng/dL. These supraphysiological peaks increased the E2 levels from baseline, but were small compared with the large increase with the T enanthate injections. Suppression of LH and lowering of sex hormone-binding globulin (SHBG)—both functions of increased and continuous serum T levels—were barely affected by sublingual T, but markedly lowered by the T enanthate.
Sublingual T was further studied to ascertain its effects when given over a longer period of time. A total of 63 hypogonadal men were divided into three groups and randomized to receiving either T enanthate, 200 mg intramuscularly (IM) every 20 days, or 2.5 or 5.0 mg of sublingual T three times per day. Mood changes were apparent in all three groups at the first follow-up visit (day 21) and persisted for the duration of the study (60 days). This included increased energy, sense of well-being, and friendliness. Negative mood parameters such as irritability and nervousness, were generally lowered in all groups, but the 2.5-mg group had the highest level of these feelings.
A separate publication by the same researchers showed that sublingual T can improve lower body muscle mass and strength (but not upper body), increase the calciotropic hormones and the bone turnover markers, and improve several aspects of sexual function. The short duration of the study (6 months) did not allow enough time for dual-energy x-ray absorptiometry (DEXA) measurements to improve. No increases in hemoglobin concentration were found, and the serum PSA went up only slightly. Maximum urine flow did decrease over the time period of the study, and one subject had to quit the study for this reason.
Despite generally positive reports with the sublingual T method, the FDA ultimately did not approve it for long-term hormone replacement in men, largely because of its pharmacokinetic profile, leading to large peak and trough variations and a very short half-life. Thus there is no commercially available sublingual T in the United States. Compounded preparations can be prepared by specially trained pharmacists, and these products have been promoted in some circles for replacement therapy, despite the previously-mentioned drawbacks. It is interesting to consider whether the sublingual T might be useful as an aid for libido and sexual function in both men and women. Its short duration of action and failure to suppress LH might actually be its strengths in this regard. Consistent with this concept, some researchers have found increases in “genital arousal” in women in as little as 45 minutes after administration. Further investigation into this possibility is needed.
Subcutaneous Testosterone Implants
Reports of T implants have been published since the late 1930s. Many different types of implants have been developed and a nearly equal number have been discarded. More recently, however, the use of Silastic capsules and crystalline T pellets has been showing promise as a method of long-term T administration. The bulk of information currently available on T implants comes from researchers and clinicians in the United Kingdom and Australia. Handelsman’s group7 in Victoria, Australia, has had extensive experience using T implants that are made by melting and molding crystalline T into 100- or 200-mg rods and inserting them using aseptic technique into the lateral abdomen at the level of the umbilicus. The group has reported over 13 years of experience and has found that it is a widely and favorably accepted method for hypogonadal men. Although extrusion of one or more of the implants from the insertion site has become the number one complication of the procedure (∼10% of procedures), more serious complications are very rare. Suppurative and/or nonsuppurative cellulitis at the site can occur in anywhere between 3 and 8% of procedures, and can usually be treated with removal of the implants. Interestingly, the likelihood of complications is almost entirely predictable based on the occupation of the patient, with more active patients having more complications.

FIGURE 19-3. The testosterone pellets, crystalline T in 100 or 200 mg rods, for long-term administration of T to hypogonadal men. When inserted into the abdomen, they provide physiologic levels of T within 1 to 2 days, T level peak at 1 month and then decline to below optimal levels at 4 to 5 months. (Courtesy of the Professional Compounding Association of America.)
The insertion of four 200-mg pellets into two or four tracks provides physiological levels of T within a day or two. Fig. 19–3 shows the testosterone pellets. The levels peak at 1 month and then gradually decrease over time so that optimal levels are not present by 4 to 5 months, requiring repeat insertion on the contralateral side. All of the usual benefits of sustained-release testosterone therapy have been demonstrated in the patients treated with this method, and no unusual problems have surfaced. The procedure has also been used in prepubertal hypogonadal boys with success. In the United States, a recent investigation into the use of the T crystalline pellets for male contraceptive has been completed and will be published in the near future (Dr. Christina Wang, Harbor-UCLA Medical Center, personal communication).
Percutaneous or Topical Testosterone
Arguably, no development in the field of androgen replacement has had the same kind of dramatic effect on clinicians and patients as the advent of topically applied T gels and creams. Although much excitement was generated with the launch of the Testoderm scrotal patch and its successor the Androderm patch, these products soon became known more for their drawbacks than their strengths. Moderate to severe rash formation in as many as 50% of its users soon put a chill on the excitement regarding transdermal therapy for hypogonadal men. Likewise, low serum T, supraphysiological levels of DHT, and the need to shave the scrotum led to low levels of acceptance of the scrotal patches.
If one thing can be said for these products, however, it is that their huge financial backing by their respective pharmaceutical companies led to some of the better and more important research as to the benefit and limitations of androgen replacement in hypogonadal men.
Thus, when Solvay released its product called Androgel in 1999, it was with a bit of skepticism that most clinicians watched to see where its Achilles heel might be. Fortunately for the population of hypogonadal men and the clinicians who treat them, Androgel has turned out to be generally all that it promised, as long as the user and prescriber look to see exactly what was promised.
Principles of Percutaneous Pharmacokinetics
By dissolving T into a lipophilic gel, one can reach concentrations up to ∼8 to 10% (wt/vol). This makes it relatively easy to apply as much as 300 mg to the skin of a potential patient. If we keep in mind that the average man needs ∼7 to 10 mg of T per day, then one can see that absorption efficiency is key with any topically applied product. By using ethanol as an enhancer and with the careful choice of other ingredients, Solvay (or the original developers, Unimed) created a product in which 50 mg applied to the skin in a 1% solution would bring physiological levels to —80 to 90% of its users. This equates with an absorption efficiency of ∼7/50 X 100 = 14%. By comparison, some of the earlier formulations that this author tried in various compounded gels or creams could only reach a 3% efficiency.
In a study published by Cutter8 in the Journal of the American Board of Family Practice, 10 hypogonadal men were selected with ages ranging from 44 to 77 years. Four of these men had newly diagnosed and six had preexisting hypogonadism. Patients were withdrawn from their previous hormone therapy and baseline laboratory studies were obtained for total testosterone, free testosterone, dihydrotestosterone, estradiol, luteinizing hormone, follicle-stimulating hormone, complete blood counts, lipid panels, and chemistry panels. The patients then started taking increasing dosages of the testosterone gel until physiological levels of testosterone were attained or until the study period of 6 weeks ended. There was no blinding, and each patient served as his own control. Testosterone and free testosterone levels were monitored weekly, and estradiol and dihydrotestosterone less frequently. At the conclusion of this study, all the baseline laboratory tests were repeated. A questionnaire evaluating the psychosexual well-being of the patients was administered before and after the treatment period. The average total testosterone level rose from 136ng/dL to 442.9ng/dL (p<.001). Average free testosterone levels rose from 34.2 pg/mL to 120.3 pg/mL (p < .001). Average dihydrotestosterone levels rose from 20.5 to 199.2 ng/dL (p = .006). Average estradiol levels rose only slightly from 34.1pg/mL to 40.0pg/mL (p = .191). Average total androgens (testosterone plus dihydrotestosterone) rose in all patients to therapeutic levels, from 149.3 ng/dL to 642.1 ng/dL (p = .001). The ratio of total androgen to estradiol rose from 5.1 to 17.1 (p < .002). Luteinizing hormone was suppressed in the six patients for whom meaningful data were available, and decreased on average from 5.66 to 1.10mIU/mL (p < .005) Lipid effects were measured, and a 15% drop in all cholesterol fractions was noted (p < .005). Evaluation of the questionnaire showed considerable improvements in sexual function and overall well-being in all but one patient. No adverse effects or nuisance problems were detected during the duration of the study. The author found that topically applied compounded testosterone gels are an effective and convenient means of hormone replacement in hypogonadal men.
Controversy of Effects of Testosterone Replacement and Prostate Cancer
A major interest today involves the effects of androgen supplementation on the prostate gland. Although T administration to hypogonadal men is usually beneficial, there is much concern regarding the potential for serious side effects resulting from T administration. Many physicians remain skeptical of the benefits of testosterone replacement therapy (TRT). It has been well established that androgen administration does not stimulate DNA synthesis (and consequently proliferation of stromal prostate cells) in normal prostates. However, the administration of T is believed to enhance any preexisting prostatic malignancy, such as prostatic carcinoma (CaP). This is based upon the following:
1. Evidence that placing rodents in hypertesto-steronemic states results in the appearance of prostatic cancer.
2. The usually dramatic responses of most human prostatic cancers to surgical or medical castration.
3. The controversial yet widely held impression that there exists a positive relationship between serum androgen levels and prostate cancer in humans.
4. The fact that androgens promote prostate cancer development in laboratory animals, which suggests that decreasing androgenic stimulation can lower prostate cancer risk.
In contrast, the following findings, particularly from human studies, refute the notion that TRT may be harmful, that hypotestosteronemia may indeed be a harbinger for CaP, and that TRT is by and large safe in the short term. Long-term data are not available, and the Institute of Medicine is in the process of commissioning one large-scale study.
1. In the literature, four studies have found a positive correlation between high serum T and risk of CaP. In contrast, six studies found that high T levels were actually associated with reduced risk and 15 studies found no difference either way.9
2. At the experimental level, a prostate cancer cell line requires initial stimulation by androgens to grow but it is eventually suppressed by androgens.10 The observation in most studies that low testosterone rather than high testosterone is associated with CaP and the subsequent suppressive effects of testosterone have led to the hypothesis that low testosterone rather than high testosterone is harmful.
3. A recent study suggested that CaP suppresses testosterone production, and may as such account for the observation that CaP is often detected in men with hypotestosteronemia.11
Discussion of the Case History
This case illustrates the difficulty that practitioners sometimes encounter with bioidentical hormone therapy. It also suggests that the practitioner be patient, and that the success of treatment can be variable. Expectations by patients should also be realistic, and a careful explanation of what might be expected should be given to them. Mr. J.J. had a large confounder, which was alcoholism. Secondary causes of hypogonadism were excluded and he was screened for a prolactinoma as well as hemochromatosis. He started off with Androgel, which was effective. However, the patient’s demands could sometimes be difficult. He wanted to try a compounded version partly out of curiosity, and also because of cost reasons. Unfortunately, the preparation produced erratic absorption. As such, it is very important to choose well-trained pharmacists such as through the Professional Compounding Association of America (PCCA). The patient subsequently went on injections, producing a “roller coaster” effect in which supraphysiological levels are reached in the initial phase, followed by subphysiological levels. Testosterone implants require a minor surgical process every 6 months, and the equipment and pellets can be obtained through PCCA.
Conclusion and Key Points
• Bioidentical hormonal therapy should be individualized. It takes time and experience to treat hypogonadal symptomatic men.
• There are presently several choices, including topical gels, injections, sublinguals, and sometimes implants.
• Oral therapy in the United States is limited, but testosterone undecanoate may prove promising when available.
• The commonly held belief that testosterone replacement in normal hypogonadal men can cause prostate cancer is untrue, but monitoring is essential.
• Before starting therapy, it is essential that the patient understand the benefits and limitations of therapy.
REFERENCES
1. Friedl KE. Effects of anabolic steroids on physical health. In: Yesalis CE, ed. Anabolic Steroids in Sports and Exercise. Champaign, IL: Human Kinetics; 1993:107–150
2. Gooren LJ. A ten-year safety study of the oral androgen testosterone undecanoate. J Androl 1994;15:212–215
3. Sih R, Morley JE, Kaiser FE, et al. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab 1997;82:1661–1667
4. Nieschlag E, Buchter D, Von Eckardstein S, et al. Repeated intramuscular injections of testosterone undecanoate for substitution therapy in hypogonadal men. Clin Endocrinol (Oxf) 1999;51:757–763
5. Gold J, High HA, Li Y, et al. Safety and efficacy of nandrolone decanoate for treatment of wasting in patients with HIV infection. AIDS 1996;10:745–752
6. Salehian B, Wang C, Alexander G, et al. Pharmacokinetics, bio-efficacy and safety of sublingual testosterone cyclodextrin in hypogonadal men: comparison to testosterone enanthate—a clinical research center study. J Clin Endocrinol Metab 1995;80: 3567–3575
7. Handelsman DJ, Mackey MA, Howe C, et al. An analysis of testosterone implants for androgen replacement therapy. Clin Endocrinol (Oxf) 1997;47:311–316
8. Cutter CB. Compounded percutaneous testosterone gel: use and effects in hypogonadal men. J Am Board Fam Pract 2001; 14:22–32
9. Slater S, Oliver RTD. Testosterone: its role in the development of prostate cancer and the potential risks from use as hormone replacement therapy. Drugs Aging 2000;17:431–439
10. Carter HB, Pearson JD, Metter EJ, et al. Longitudinal evaluation of serum androgen levels in men with and without prostate cancer. Prostate 1995;27:25–30
11. Zhang PL, Rosen S, Veeramachaneni R, et al. Association between prostate cancer and serum testosterone levels. Prostate 2002;53:179–182
< div class='tao-gold-member'>









