7
Alzheimer’s Disease in Older Men: Are There Gender-Specific Etiological Issues and Treatments?
Case History
The classic thinking is that testosterone is an androgenic hormone responsible for normal growth and development of male sex and reproductive organs. It is produced from the testes and also converted from dehydroepiandrosterone (DHEA) in women. There is increasing evidence that androgens may modulate muscle growth, function, and possibly cognitive function as well. We applied this hypothesis to a patient we treated recently in an Alzheimer’s center. This was a patient admitted to our Alzheimer’s center a year ago for terminal care. The patient had a history of advanced Alzheimer’s disease (AD) with associated agitation and delusion behavior. He also had asthma and diabetes mellitus type 2. On admission the patient seemed distant, and he did not answer any questions. On initial physical examination the patient weighed 138 pounds. The initial Mini–Mental State Examination (MMSE) score was 0/30 and the initial total testosterone 160 ng/dL [240–1000 ng/dL]. After informed consent from the family, he was started on an anabolic steroid primarily to increase functionality, but his cognitive parameters were observed.
Fig. 7–1 shows that after implementation of testosterone the MMSE score improved from 0/30 to 5/30, the clock drawing test (CDT) from 0/4 to 1/4, and the Tinetti scale, a fall assessment tool, from 5/2 to 20/28. This case study demonstrates that the use of testosterone in selected AD patients may possibly help with the improvement of cognition and functionality. AD is a terminal disease with little hope of improvement and any treatment modality that would improve the quality of life of a patient is to be investigated further. This patient tolerated the treatment well. It is difficult to conclude if the testosterone treatment alone or the good supportive care resulted in the improvement of the patient.
Do Men Have a Lower Probability of Alzheimer’s Disease and How Can Men Protect Themselves Against It?
To the casual observer, it seems that AD is less common among men. A walk through a nursing home where AD is prevalent would more likely result in an encounter with more female residents than male residents. Men on average live 7 years less than women, and this may account for the seemingly higher prevalence of AD in women, as women tend to live longer and AD is a function of age.1 However, a recent study conducted in Rochester argues that AD may be as common in men as in women if corrected for the age differences,2 though gender-specific risk factors for AD cannot be excluded. For instance, smoking in men has been shown to increase the risk of AD.3 Likewise, HIV-positive men who are carriers of the e4 allele of apolipoprotein E (ApoE) have an increased risk of AD.4
The risk of AD in men may be reduced by several factors such as the use of antioxidants, moderate alcohol consumption, and exercise.5,6 The Honolulu Heart Study examined 3385 men, and those who prophylactically consume vitamins C and E had a 88% reduction in risk for AD.5 Another study from the Netherlands suggests that moderate alcohol intake may also decrease the risk for AD in men.6 Several epidemiological studies have suggested that lifestyle is important in reducing risk of AD in men. For example, Honolulu Asia Aging Study found that men who walked and socialized regularly exhibited an 80% decreased risk of AD.5
Altered neuroendocrine regulation of cognition may also predispose men to AD.7 Men, when they age beyond 50 years may suffer from androgen decline, sometimes termed andropause or androgen decline in aging males (ADAM).8 This decline in androgen occurs more gradually as compared with women in menopause. The decline is more marked with bioavailable testosterone than with total testosterone, partly because of the changing affinity of sex hormone–binding globulin (SHBG) with aging.7 It has been observed that older men who have undergone androgen replacement often report improved memory function or report feeling “brighter.” This suggests that androgen replacement may play an important role in improving memory in men entering into a transitional stage of lowered hormones. Low levels of androgen are not restricted to risk of AD per se but have also been reported for risk of vascular dementia.9–11 Androgen replacement therapy was associated with improved cognition in vascular dementia patients.12–14
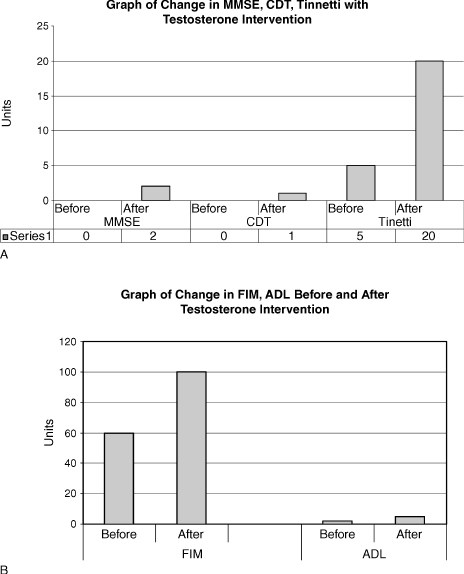
FIGURE 7-1. (A) Change in mini–mental state examination (MMSE), clock drawing test (CDT), and Tinneti (scale used to measure risk for falls) with testosterone intervention, and (B) change in activities of daily living (ADL) and functional instrument measure (FIM) before and after intervention with testosterone.
Alzheimer’s disease is thought to occur less frequently in men than in women, and as such, it may seem logical to infer that androgens, which are more abundant in men, may be protective. This hypothesis has been tested in animal as well as human models, and indeed there may be a link. The following sections highlight some of the supportive evidence. AD is such a complex disease that it would be imprudent to attribute the association to be direct.
Another piece of the puzzle has been the observation that gonadotrophins such as the luteinizing hormone (LH) and the follicle-stimulating hormone (FSH) tend to be elevated in AD, and it is unclear if the hypogonadal state results in a rise in these gonadotrophins. Attempts have been made to use antigonadotrophins to treat AD, but this may sound irrational, as there might be further induction of the hypogonadal state, which in itself is suspected to be a risk factor for AD. As mentioned, AD remains a complex illness and has many facets yet to be defined. To date, the use of acetyl cholinesterase inhibitors for AD has not been very successful in terms of a cure. As such, it is prudent to prod other possibilities for therapeutics, as the disease is relentless and devastating.
Presently Recognized Drug Treatments for Alzheimer’s Disease
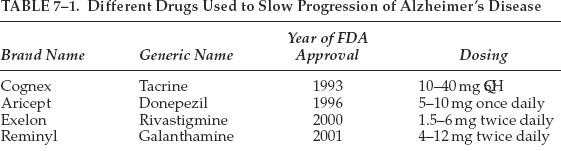
As of this writing, there are five drugs that have been approved in the United States by the Food and Drug Administration (FDA) for treating the cognitive symptoms of Alzheimer’s disease: memantine, galanthamine, rivastigmine, donepezil, and tacrine. Tacrine needs close liver function monitoring. These drugs belong to the class of cholinesterase inhibitors. Each acts in a different way to delay the breakdown of acetylcholine. It is believed that acetylcholine in the brain facilitates communication among nerve cells and is thus important for memory. It is not possible to measure patients’ level of acetylcholine and as such treatment by and large is empirical and based on a clinical diagnosis. The reader is referred to other texts for a more detailed discussion of these treatments. Table 7–1 summarizes the drugs used to treat progression of Alzheimer’s disease.
In general, these drugs are most effective when treatment is begun in early stages. They have all been shown to modestly slow the progression of cognitive symptoms and reduce problematic behaviors in some people. Unfortunately, at least 50% of patients do not respond to the acetylcholinesterase inhibitors. Although the effect of these medications is modest, studies show that when they do work, they can make a significant difference in a person’s quality of life and day-to-day functioning (activities of daily living, ADL). The main differences between the drugs are the side effects they produce and the number of times they must be taken daily (Aricept is taken once daily, Exelon and Reminyl are taken twice a day).
One of the new treatments FDA approved for treatment of cognitive problems is memantine. Overstimulation of the N-methyl-D-aspartate (NMDA) receptor by glutamate is implicated in neurodegenerative disorders. In a double-blind, placebo-controlled trial, patients receiving memantine had a better outcome than those receiving placebo.15 Memantine was not associated with a significant frequency of adverse events. Antiglutamatergic treatment reduced clinical deterioration in moderate-to-severe AD, a phase associated with distress for patients and burden on caregivers, for which other treatments are not available. Many drugs as well as nutraceuticals have been studies including vitamin E and gingko biloba, both of which look promising.
Laboratory Models of Androgens and Brain Function
Estrogens have been thought to be protective against AD in women, but treatment of AD patients with estrogens has by and large not been successful.16 Animal studies have indicated that testosterone does play an important role in cognition. Many researchers have postulated that the action is through the androgen receptors (ARs). It has been discovered that AR and its associated messenger RNA is abundant in the rat brain. The cerebral cortex and hippocampus are areas that are rich in ARs.17 In one study the blockade of ARs in mice expressing the major AD genetic risk factor, the ∊4 of apolipoprotein E (ApoE 4) resulted in the development of prominent deficits in spatial learning and memory. Paradoxically, female neuron-specific enolase (NSE)—ApoE4 mice, which are prone to impairments in spatial learning and memory, exhibited memory improvement in response to androgen treatment (Fig. 7–2).18 In addition, cytosolic AR levels were decreased in NSE-ApoE4 mice and improved memory treatment in the NSE-ApoE4 female mice was associated with increased cytosolic AR levels.
Reproductive hormones influence the development of the central nervous system (CNS). For instance, androgens influence neuritic arborization and the receptive field of individual cells, whereas estrogen induces this communication by forming spines, synapses, and gap junctions. As such, estrogens and androgens act in different but complementary ways to modulate neural development and organization.19 Castration of animals serves as a model to observe cognitive changes. For example, it has been shown that sexually intact male dogs were significantly less likely than neutered dogs to progress from mild to severe impairment.20 These observations are consistent with the notion that circulating testosterone in aging sexually intact male dogs slows the progression of cognitive impairment. The senescence-accelerated mouse (SAMP) exhibits age-related learning and memory deficits in the ability to perform inferential tasks.21 These memory deficits are associated with a corresponding decline in testosterone levels with age. Testosterone replacement improved age-related impairment of learning and memory.22 It has been postulated that cognitive impairment of SAMP8 mice may be related to an interaction of aging and lowered testosterone levels.
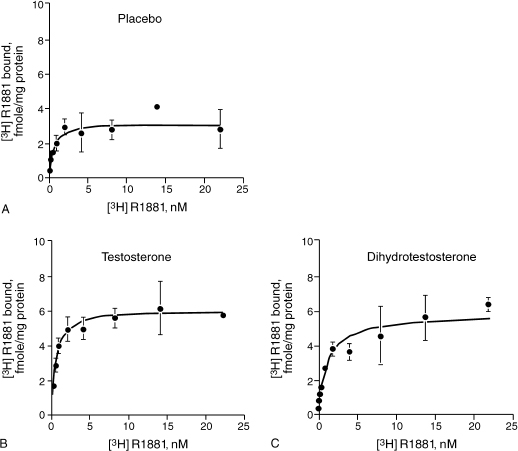
FIGURE 7-2. Effect of (A) placebo, (B) testosterone, and (C) dihydrotestosterone on androgen receptor (AR) saturation curves in female NSE-ApoE4 mice. Testosterone and dihydrotestosterone effects on androgen receptors, including those in the brain, are more significant than the placebo was. (Adapted from Raber J, Bongers G, Le Fevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. J. Neurosci 2002;22:5204–5209, with permission.)
Intracellular neurofibrillary tangle formation as a result of tau protein hyperphosphorylation is one of the major neuropathological hallmarks of AD. It has been demonstrated that heat shock–induced hyperphosphorylation of tau protein in the brain of orchiec-tomized male rats can in fact be reduced by testosterone.23 Interestingly, this effect was not seen with estrogens, indicating it was a direct effect of testosterone or its metabolite dihydrotestosterone. It has also been demonstrated that treatment with testosterone decreases the secretion of amyloid β (Aβ) peptide from cerebrocortical neurons of rats.24 Evidence is accumulating to indicate that changes in Aβ precedes tau hyperphosphorylation.25 In addition to reducing Aβ levels, testosterone and dihydrotestosterone can protect cultured hippocampal neurons of rats from cell death by directly blocking Aβ toxicity via an estrogen-independent mechanism.26 Testosterone also exerts its neuroprotective effects through receptor-mediated mechanisms27,28 by stimulating increased secretion of a metabolite of amyloid precursor protein (sAPP),29 which has been demonstrated to exhibit neurotrophic properties.
Dehydroepiandrosterone sulfate (DHEAS) is synthesized in situ in the brain. Therefore, it has been termed a neurosteroid. Numerous animal studies have demonstrated the neuroprotective and memory-enhancing effect of DHEAS. DHEAS concentrations decline with age in various animals, including the rhesus monkey.30 The data on DHEA and cognition is conflicting. One study demonstrated that cognitively impaired and unimpaired aged rhesus monkeys did not differ in their DHEAS levels.31 DHEA replacement enhanced memory in aged mice in several experiments.27–29 In experiments, DHEA has been shown to significantly reverse pharmacologically induced neurotoxicity with NMDA, dimethylsulfoxide kainic acid, H2O2, dizocilpine, ethanol and scopolamine.4,32–34 Possibly, DHEA may be useful in treating memory impairment based on its antioxidant and neuroprotective effects in the hippocampus. It has been reported that DHEA increased the production of amyloid precursor protein (APP) and promoted the release of its metabolite sAPP from PC12 cells.35,36 Although the increased production of APP per se may lead to acceleration of mild cognitive disorder presumably as a result of increased Aβ production, the increased APP produced in DHEAS-treated cells is processed through the non-amyloidogenic pathway to release APP, which is known to have neuroprotective properties that may be mediated in part by its ability to promote neurite outgrowth.36–38 The effects of DHEA on cognition may be mediated through its nongenomic action on several neurotransmitter receptors, including cholinergic neurons.39 Despite the promising data from animals, the effects of androgens on human cognition are less clear and will be discussed in the following sections.
Examining Human Studies of Androgens and Cognition
Older men often complain of memory problems. A Texas study of 302 older men found that 36% of patients identified themselves as experiencing andropause on a standardized questionnaire survey and reported memory loss as a symptom.40 Another study in Perth, Western Australia, which employed a similar questionnaire given to 100 men treated for low testosterone, found initial symptoms included memory loss and lapses in concentration (85%), fatigue (96%), mood problems (83%), and loss of libido (77%) (Linda Byart, personal communication). Hormone replacement therapy in this cohort resulted in 86% of men mentioning an improvement in memory and attention span.
Investigators have examined the effect of androgen substitution or withdrawal upon various study populations. By and large, these studies are limited by population size, but are very encouraging. A double-blind study conducted by Janowsky et al41 demonstrated that transdermal testosterone enhanced spatial cognition of healthy men aged 60 to 75 years when testosterone levels were raised for a 3-month period to those commonly found in young men. Endogenous production of estradiol was decreased in men receiving testosterone supplementation, and estradiol levels correlated inversely to performance on tests of spatial cognitive skills.25 A 6-week randomized, double-blind, placebo-controlled study of healthy older men aged 50 to 80 years found that 100 mg of testosterone enanthate improved both spatial and verbal memory.42 Another small randomized, double-blind study of healthy volunteers aged 61 to 75 years demonstrated improved working memory following intramuscular injections of testosterone enanthate 150mg/week. Better performance on tasks involving frontal lobe–mediated working memory was related to a higher testosterone-to-estrogen ratio.28 The subjects in these studies were healthy volunteers who did not have AD.
Studies involving testosterone substitution have historically been confounded by concurrent changes in estradiol levels and include patient samples that may not be generalized to the population of elderly male patients experiencing low-testosterone syndrome. A 12-month randomized, controlled trial of 32 hypogonadal men (bioavailable testosterone <60 ng/dL), aged 51 to 75 years, failed to demonstrate any difference in verbal or nonverbal memory with 200 mg testosterone cypionate biweekly; however, visuospatial ability was not specifically examined. The authors hypothesized that their inability to demonstrate the more generalized effects on cognition may have been due to the rise in estradiol levels in the treatment group.43
Sexual reassignments, because of the use of sex steroids, gave an opportunity for researchers to observe the effects of hormones on brain function. For instance, Slabbekoorn et al44 demonstrated a profound effect of androgen treatment on spatial ability in female-to-male transsexuals (FMs) over a period of 18 months. After 3 months of cross-sex testosterone treatment, 25 FMs demonstrated a significant improvement on a three-dimensional rotated figures task of spatial ability. Untreated male-to-female transsexuals (MFs) had higher scores on visuospatial tasks than untreated FMs transsexuals.44
Recently, in a small 8-week randomized, controlled trial, testosterone was shown to exhibit a curvilinear dose-response effect for different verbal and spatial cognitive functions. Supraphysiological levels of testosterone (200 mg testosterone enanthate/week) reduced visuospatial ability and improved verbal fluency in 30 healthy young men aged 19 to 45 years.45 This finding confirms a previous study that showed a curvilinear relationship between spatial performances and circulating testosterone concentrations with optimal effects on spatial performance, associated with intermediate plasma levels of testosterone.38 This is consistent with the hypothesis first proposed by Janowsky et al41 that high levels of testosterone may be aromatized to estradiol within the brain.
The development of new imaging technology has enabled investigators to explore neuropharmacology in a functional manner. A small study of six men with hypogonadotropic hypogonadism when given testosterone demonstrated enhanced cerebral glucose metabolism as assessed by using F18-deoxyglucose positron emission tomography (PET) with increased visuospatial capacity during a three-dimensional mental rotation task.46 Brain localization on PET was consistent with those areas that were observed to be activated by such tasks in a previous study on eugonadal men.23
The hypothesis that gonadal replacement therapy might prevent or delay AD in postandropausal men has recently been supported by a study of the effect of gonadal hormone withdrawal on the levels of plasma (Aβ. This 4-kd peptide is the major protein component of the extracellular amyloid plaques, which is a neuropathological hallmark of AD47 and is thought to play a key role in its pathogenesis.48 Plasma Aβ levels increased in men with prostate cancer in response to flutamide (250 mg, three times daily) and leuprorelin acetate (22.5 mg, weekly for 12 weeks) treatment to lower plasma testosterone. This increase in plasma Aβ paralleled a rapid decrease in both plasma testosterone and 17β-estradiol, and remained elevated over a 6-month period while gonadal hormones remained at low detectable levels (Fig. 7–3).49 However, regulation of Aβ production represents only one of several potential mechanisms that need evaluation. Some of these other potential candidate mechanisms are based on testosterone’s ability to block neuronal apoptosis50 as well as indirectly to prevent tau phosphorylation,23 to increase the production of the neurotrophic agent nerve growth factor51 and insulin growth factor-1,52 and to reverse the age-related increases in glial fibrillary acidic protein (GFAP).53 Increased GFAP reflects activation of astrocytes, which have been implicated in playing a more prominent role in the pathogenesis of AD.54
The adrenal steroid hormone DHEA is known to decrease with aging and is commonly used as an anti-aging nutritional supplement. Clinical studies of the effects of DHEA and its sulfate DHEAS on cognition have produced conflicting results. Fifty-two community-dwelling AD patients (mean age 76 years) were compared with a control group of age- and gender-matched healthy elderly men and women on a test of everyday memory. No differences were seen between the AD patients and controls in DHEAS levels. However, AD patients with higher levels of DHEAS scored better than those with lower levels.55 A longitudinal study of 883 community-dwelling men (mean age at entry 53 years, range 22–91 years) was conducted for as long as 31 years (mean 11.5 years), with biennial assessments of multiple cognitive domains. Neither the rates of decline in mean DHEAS nor the mean DHEAS concentrations within individuals were related to cognitive status or cognitive decline.56 It should be noted, though, that most of the previous studies are relatively small, and a definitive outcome must await properly controlled large-scale studies where dose effects are also evaluated, as recently reported by Racchi et al.57
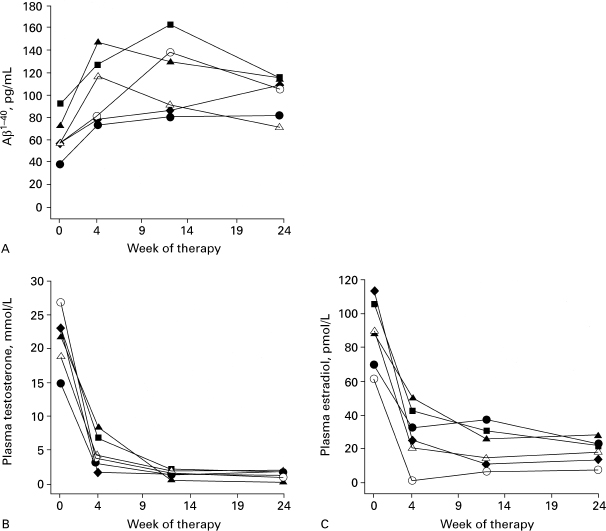
FIGURE 7-3. Plasma concentration of (A) β amyloid, (B) testosterone, and (C) estradiol before and after flutamide and leuprorelin acetate treatment. (Adapted from Gandy S, Almeida OP, Fonte J, et al. Chemical andropause and amyloid-B peptide. JAMA 2001;285:2195–2196, with permission.)
A cross-sectional sample of 63 frail elderly patients aged 66 to 103 years (mean age 86 years) at a multilevel care facility actually found an opposite relationship to that postulated between DHEAS and cognitive impairment measured as performance on the Blessed Memory Information Concentration Test (BMIC). These findings suggest that the relationships between steroid hormones of the hypothalamic-pituitary axis may be different in the elderly nursing home population than in other groups, though this relatively small study needs verification with a larger cohort.
Antigonadotrophin Therapy for Alzheimer’s Disease
The finding that gonadotrophins may be raised in AD has led to the possibility of using antigonadotropins such as leuprolide in the treatment of AD.58 In individuals with AD, there is a twofold elevation in the serum concentrations of the gonadotropins, LH, and FSH compared with age-matched controls. Whether elevated gonadotropins actually cause AD or merely represent a feedback response to low testosterone levels remains to be determined. However, we have recently found that LH does promote increased Aβ secretion in cultured neurons (G. Verdile, C. Atwood, and R. Martins, unpublished results). LH has been localized in the cytoplasm of pyramidal neurons where it is found to be increased together with neurofibrillary tangles in AD brain compared with age-matched control brain. Although the functional consequences of increased neuronal LH are unknown, it is notable that LH is primarily localized to those neurons that are known to be vulnerable to AD-related neurodegeneration. Elevated serum and cortical neuron levels of LH, coupled with the decline in sex steroid production, could play important roles in the pathogenesis of AD. However, if a role for LH in AD is established, leuprolide treatment per se, while reducing LH, would also further deplete the already low testosterone levels, which are important for both reducing Aβ levels and simultaneously increasing the levels of the neurotrophic factor, sAPP. Thus testosterone replacement would be needed to complement the protective effect of leuprolide.
Discussion of Case History
Currently, there is no recommendation of the use of androgens for AD, and it is not FDA approved. The treatment of this patient with advanced AD was based on his hypogonadal state. The aim was to increase functionality with an anabolic hormone, and offer him a better quality of life. The observed improvement in cognition was a bonus, and he did become more communicative. He had improvements in his MMSE and CDT (which is a measure of visual-spatial skills). His functionality as measured by the functional instrument of measure (FIM) scale and the ADL scale also improved. Paradoxically, it has been reported in the literature that sexually disruptive male AD patients can benefit from estrogen treatment. This patient did not turn hypersexual, but that has been noted to occur in some AD patients. Carefully monitoring is needed.
Conclusion and Key Points
Many men become hypogonadal as a result of aging, although some men exhibit changes as early as their 40s. It has been established that androgen replacement may help restore energy and libido. An added major benefit may be the restoration of cognitive function. Many patients with advanced AD also suffer from the wasting syndrome, which can lead to falls. Testosterone being an anabolic steroid may help restore functionality in these patients.
• AD is seemingly less common in men because of the shorter life span of men, and AD is a function of aging.
• Men can reduce their risk of AD by consuming vitamin C and E.
• Moderate alcohol, exercise, and socializing may also decrease the risk for AD.
• In in vitro studies, androgens increase neuritic arborization and the receptive field of cells, whereas estrogens induce communication between cells.
• There is a link between amyloid and androgens, which suggests that AD is linked to androgens.
• Small clinical studies have suggested that cognition can be improved with androgens, in particular the visual spatial domain.
REFERENCES
1. Kranczer S. Continued U.S. longevity increases. Stat Bull Metrop Insur Co 1999;80:20–27
2. Edland SD, Rocca WA, Petersen RC, Cha RH, Kokmen E. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester, Minn. Arch Neurol 2002;59:1589–1593
3. Ott MM, Breteler, Harskamp F, Stijnen T, Hofman A. Incidence and risk of dementia. The Rotterdam Study. Am J Epidemiol 1998;147:574–580
4. Corder EH, Robertson K, Lannfelt L, et al. HIV-infected subjects with the E4 allele for APOE have excess dementia and peripheral neuropathy. Nat Med 1998;4:1182–1184
5. Masaki KH, Losonczy KG, Izmirlian G, et al. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology 2000;54:1265–1272
6. Ruitenberg A, van Swieten JC, Witteman JC, et al. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet 2002;359:281–286
7. Vermeulen A, Kaufman JM. Diagnosis of hypogonadism in the aging male. Aging Male 2002;5:170–176
8. Morley JE, Perry HM III. Androgen deficiency in aging men. Med Clin North Am 1999;83:1279–1289
9. English KM, Mandour O, Steeds RP, Diver MJ, Jones TH, Channer KS. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur Heart J 2000;21:890–894
10. Pandey KN, Oliver PM, Maeda N, Smithies O. Hypertension associated with decreased testosterone levels in natriuretic peptide receptor-A-gene-knockout and gene-duplicated mutant mouse models. Endocrinology 1999;140:5112–5119
11. Rolf C, Nieschlag E. Potential adverse effects of long-term testosterone therapy. Baillieres Clin Endocrinol Metab 1998;12: 521–534
12. Azuman T, Nagai Y, Saito T, Fanauchi M, Matsubara TS. The effect of dehydroepiandrosterone sulfate administration to patients with multi-farct dementia. J Neurol Sci 1999; 162:69–73
13. Sih R, Morley J, Kaiser FE, Perry IHM, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab 1997;82:1661–1667
14. Webb CM, Adamson DL, De Zeigler D, Collins P. Effect of acute testosterone on myocardial ischemia in men with coronary artery disease. Am J Cardiol 1999;83:437–439
15. Reisberg B, Doody R, Stottler A, et al. Memantine in moderate to severe Alzheimer’s disease. N Engl J Med 2003;348: 1333–1341
16. Grady D, Yaffe K, Kristof M, Liu F, Richards C, Barrett-Connor E. Effect of postmenopausal therapy on cognitive function: the Heart and Estrogen/Progestin Replacement Study. Am J Med 2002;113:543–548
17. Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 1990;294:76–95
18. Raber J, Bongers G, Le Fevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. J Neurosci 2002;22:5204–5209
19. Lustig RH. Sex hormone modulation of neural development in vitro. Horm Behav 1994;28:383–395
20. Hart BL. Effect of gonadectomy on subsequent development of age-related cognitive impairment in dogs. J Am Vet Med Assoc 2001;219:51–56
21. Ohta A, Akiguchi I, Seriu NK, et al. Deterioration in learning and memory of inferential tasks for evaluation of transitivity and symmetry in aged SAMP8 mice. Hippocampus 2002;12: 803–810
22. Flood JF, Farr S, Kaiser FE, La Regina M, Morley JE. Age-related decrease of plasma testosterone in SAMP8 mice: Replacement improve age-related impairment of learning and memory. Physiol Behav 1995;57:669–673
23. Papasozomenos SC. The heat shock-induced hyperphosphorylation of tau is estrogen-independent and prevented by androgens: implication for Alzheimer disease. Proc Natl Acad Sci USA 1997;94:6612–6617
24. Gouras GK, Xu H, Gross RS, et al. Testosterone reduces neuronal secretion of Alzheimer’s β-amyloid peptides. Proc Natl Acad Sci USA 2000;97:1202–1205
25. Lewis J, Dickson DW, Lin WL, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP Science 2001;293:1487–1491
26. Pike CJ. Testosterone attenuates β-amyloid toxicity in cultured hippocampal neurons. Brain Res 2001;919:160–165
27. Flood JF, Morley JE. Learning and memory in the SAMP8 mouse. Neurosci Biobehav Rev 1998;22:1–20
28. Frye CA, Lacey EH. The neurosteroids DHEA and DHEAS may influence cognitive performance by altering affective state. Physiol Behav 1999;66:85–92
29. Goodenough S, Engert S, Behl C. Testosterone stimulates rapid secretory amyloid precursor protein from rat hypothalamic cells via the activation of the mitogen-activated protein kinase pathway. Neurosci Lett 2000;296:49–52
30. Kemnitz JW, Roecker EB, Haffa AL, et al. Serum dehydroepiandrosterone sulfate concentrations across the life span of laboratory-housed rhesus monkeys. J Med Primatol 2000; 29:330–337
31. Herndon JG, Lacreuse A, Ladinsky E, Killiany RJ, Rosene DL, Moss MB. Age-related decline in DHEAS is not related to cognitive impairment in aged monkeys. Neuroreport 1999;10: 3507–3511
32. Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J. Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci USA 1998;95:1852–1857
33. Reddy DS, Kulkarni SK. Possible role of nitric oxide in the nootropic and antiamnesic effects of neurosteroids on aging-and dizocilpine-induced learning impairment. Brain Res 1998; 799:215–229
34. Shen S, Cooley DM, Glickman LT, Glickman N, Waters DJ. Reduction in DNA damage in brain and peripheral blood lymphocytes of elderly dogs after treatment with dehydroepiandrosterone (DHEA). Mutat Res 2001;Sep 1:480–481
35. Danenberg HD, Haring R, Heldman E, et al. Dehydroepiandrosterone augments M1-muscarinic receptor-stimulated amyloid precursor protein secretion in desensitized PC12M1 cells. Ann N Y Acad Sci 1995;774:300–303
36. Danenberg HD, Haring R, Fisher A. Dehydroepiandrosterone (DHEA) increases production and release of Alzheimer’s amyloid precursor protein. Life Sci 1996;59:1651–1657
37. Milward EA, Papadopoulos R, Fuller SJ, et al. The amyloid protein precursor of Alzheimer’s disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron 1992;9:129–137
38. Sisodia SS, Koo EH, Beyreuther K, Unterbeck A, Price DL. Evidence that β-amyloid protein in Alzheimer’s disease is not altered by normal processing. Science 1990;248:492–495
39. Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and its sulfate in the central nervous system: effect on cognition and emotion in animals and humans. Brain Res Brain Res Rev 1999;30:264–288
40. Tan RS. Memory loss as a reported symptom of andropause. Arch Androl 2001;47:185–189
41. Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behav Neurosci 1994;108: 325–332
42. Cherrier MM, Asthana S, Plymate S, et al. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology 2001;57:80–88
43. Morrison MF, Redei E, TenHave T, et al. Dehydroepiandrosterone sulfate and psychiatric measures in a frail, elderly residential care population. Biol Psychiatry 2000;47:144–150
44. Slabbekoorn D, van Goozen SHM, Megens J. Activating effects of cross-sex hormones on cognitive functioning: a study of short-term and long-term hormone effects on transsexuals. Psychoneuroendocrinology 1999;24:423–447
45. O’Connor DB, Archer J, Hair WM, Wu FC. Activational effects of testosterone on cognitive function in men. Neuropsychologia 2001;39:1385–1394
46. Zitzmann M, Weckesser M, Schober O, Nieschlag E. Changes in cerebral glucose metabolism and visuospatial capacity in hypogonadal males under testosterone substitution therapy. Exp Clin Endocrinol Diabetes 2001;109:302–306
47. Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA 1985; 82:4245–4249
48. Martins RN, Robinson PJ, Chleboun JO, Beyreuther K, Masters CL. The molecular pathology of amyloid deposition in Alzheimer’s disease. Mol Neurobiol 1991;5:389–398
49. Gandy S, Almeida OP, Fonte J, et al. Chemical andropause and amyloid-B peptide. JAMA 2001;285:2195–2196
50. Lue YH, Sinha Hikin AP, Swerdloff RS, et al. Single exposure to heat induces stage-specific germ cell apoptosis in rats: role on intratesticular testosterone on stage specificity. Endocrinology 1999;140:1709–1717
51. Tirassa P, Thiblin I, Agren G, Vigneti E, Aloe L, Stenfors C. High-dose anabolic steroids modulate concentration of nerve-growth factor and expression of its low affinity receptor (p75-NGFr) in male rat brain. J Neurosci Res 1997;47:198–207
52. Wyss-Coray T, Loike JD, Brionne TC, et al. Adult mouse astrocytes degrade amyloid-β in vitro and in situ. Nat Med 2003;9:453–457
53. Day JR, Frank AT, O’Callaghan JP, Jones BC, Anderson JE. The effect of age and testosterone on the expression of glial fibrillary acidic protein in the rat cerebellum. Exp Neurol 1998; 151:343–346
54. Yoshizawa A, Clemmons DR. Testosterone and insulin-like growth factor (IGF) I interact in controlling IGF- binding protein production in androgen-responsive forsekin fibroblast. J Clin Endocrinol Metab 2000;85:1627–1633
55. Carlson LE, Sherwin BB, Cherkow HM. Relationships between dehydroepiandrosterone sulfate (DHEAS) and cortisol (CRT) plasma levels and everyday memory in Alzheimer’s disease patients compared to healthy controls. Horm Behav 1999;35: 254–263
56. Moffat SD, Zonderman AB, Harman SM. The relationship between longitudinal declines in dehydroepiandrosterone sulfate concentrations and cognitive performance in older men. Arch Intern Med 2000;160:2193–2198
57. Racchi M, Balduzzi C, Corsini E. Dehydroepiandrosterone (DHEA) and the aging brain: flipping a coin in the fountain of youth CNS. Drug Rev 2003;9:21–40
58. Bowen RL, Smith MA, Harris PL, et al. Elevated luteinizing hormone expression colocalizes with neurons vulnerable to Alzheimer’s disease pathology. J Neurosci Res 2002;70: 514–518
< div class='tao-gold-member'>