2
Delaying Aging for Men: Longevity Lessons from Women
Case History
A 58-year-old college professor, who was considering retirement. He was happily married to his wife of 30 years, who currently was postmenopausal and on estrogens. He had heard that good preventive medicine could be one of the keys for life extension. He had had annual physicals since he was 45 years old and was generally well. He did not smoke but drank a glass of wine every night. He admitted to exercising infrequently. His main aim was to remain healthy through his “golden years,” and he was inquiring about possible interventions including diet, exercise, and hormones. He jokingly said that he wanted to live as long as his wife, who was 5 years younger than he. He requested to have blood drawn for evaluation. He realized that most of these tests would not be covered by insurance, but he wanted to proceed nonetheless. Physical examination revealed that he was 5 feet 7 inches, weighed 151 pounds, and had a body fat content of 23% and blood pressure (BP) of 120/85 mm Hg. He also had a mild degree of gynecomastia and normal testicular size. He did have some hair loss over his scalp and thinning of his skin, relative to his age. He was asked to catch a falling ruler with his fist to test his reflexes, and he did well on that test.
Significant laboratory results included: fasting glucose 96mg/dL, cholesterol 183mg/dL, triglyceride 180mg/dL, hemoglobin HbA1C 5.1, insulin-like growth factor I (IGF-I; somatomedin C) 83ng/mL (range 90–360), total testosterone 273 ng/dL (range 260–1000), free testosterone 47.5pg/mL (range 50–210), prolactin 5ng/mL, dehydroepiandrosterone sulfate (DHEAS) 429 µg/dL (range 20–413), thyroid-stimulating hormone (TSH) 2.05 µIU/mL, free triiodothyronine (T3) 3.2pg/mL (range 2.3–4.2), free thyroxine (T4) 1.0ng/dL (range 0.8–1.8), luteinizing hormone (LH) 2.2mIU/mL, estradiol 8pg/mL (range 10–50), insulin 21 µU/mL (range <20), homocysteine 12.9 µmol/L (range 5.4–11.4), prostate-specific antigen (PSA) 0.4 ng/dL.
The results were discussed with him, as it was noted that he had marginally low free testosterone and IGF-I. He also had marginally high homocysteine levels. He inquired about hormone replacement with testosterone and human growth hormone. He also asked for intervention to bring his homocysteine levels down, which he had heard was a risk factor for cardiac disease. He inquired about whether he should change his diet and if exercise would alter some of his biomarkers.
An Overview of Aging
In the United States, average life expectancy at birth is ∼79 years for women and ∼72 years for men. The oldest person for whom reliable records exist was a woman who recently died in France at the age of 123. The odds of someone living this long are about one in 6 billion. From a practical point of view, we can consider a century as the average maximum of human life. We are not there yet, of course. At present, average life expectancy for those born after 1960 is ∼85 years.
Before learning about delaying aging, it is imperative that one understands the processes of aging and the limitations of human life expectancy. The process of aging is universal for all forms of life. It begins at birth, or arguably on conception. Since time began, men have tried to go against aging, and to search for immortality. In reality, at the microscopic level, the accumulation of the diverse deleterious changes produced by aging in cells and tissues progressively impair function and can eventually cause death. Aging, in general, can be attributed broadly to the following five categories:
• Genetic defects
• Development
• Disease states
• The environment
• An inborn process—the aging process
The probability of death at a specific age can be said to be a measure of the average number of aging changes accumulated by persons of the physiological age and the rate of change of this measure as the rate of aging. Overall, the probability of death is decreased by improvements in general living conditions, preventive medicine, immunizations, and good habits like abstinence from smoking and drinking alcohol in moderation. During the past 2000 years, it is amazing that the average life expectancy at birth (ALE-B) of the human race has risen from 30 years in the Roman Empire to almost 80 years today in the developed countries. This is unprecedented in history. Most of the increase in ALE-B is attributed not so much to advances in medicine but to the general improvement of economies and perhaps to immunizations. Probabilities of death in the developed countries are now near limiting values, and ALE-B is approaching plateau values. Overall, one can argue that there is no limit, but biologically as a species we are limited to ∼85 years in general. In the United States, we are thus on average 6 to 9 years less than the potential maximum at this point. Obviously, there are variations from person to person.
In general, the inherent aging process now largely determines the chances of death after the age of 28 years. It is interesting to note that, in Sweden, only 1.1% of female cohorts die before this age; the remainder die off at an exponentially increasing rate with advancing age. The inherent aging process limits ALE-B to around 85 years, and the maximum life span (MLS) to ∼122 years.
Past efforts to increase ALE-B did not require an understanding of the biological processes of aging. Such knowledge will be necessary in the future to significantly increase ALE-B and MLS. This knowledge is also required to satisfactorily plan for the medical, economic, and social problems associated with advancing age. For instance, many developed countries are increasingly burdened by social problems and increasing taxation as the population pyramid changes from one with a larger base of young people to one with a larger base of older people.1
There are many theories that have been proposed to account for aging, and they should be used to the extent they are feasible. The difficulty of the science of delaying aging or anti-aging principles is that often these theories are built on animal models, and they may or may not apply to human beings. Overall, the previous measures evolved by societies to ensure adequate care for older individuals are rapidly becoming inadequate because of changes in lifestyle, the growing percentage of older people, declining fertility rates, and the diminishing size of the workforce to provide for the elderly. Measures are being advanced to help with this problem but they largely address the preventive level, where lifestyle changes can significantly impact longevity. Prospects are bright for further increases in the span of functional life and improvements in the quality of lives of older individuals.
Limits to Human Longevity
As mentioned previously, the human life span at this point is limited to ∼122 years and average life expectancy to 85 years in developed countries, although there is a predilection for longevity in women. However, demographic approaches to modeling and forecasting mortality are often based on the observation of short-term trends in death statistics and the assumption that future mortality will exhibit patterns similar to those of the recent past. This extrapolation method has led some demographers to conclude that ALE-B in the near future may reach 100 years.
Similar predictions follow from other demographic models that establish a hypothetical link between risk factor modification and changes in death rates. Risk factor modification would include attention to diet, smoking cessation, and exercise. These predictions are examined within the context of the observed mortality records and their biological plausibility assessed based on the current theories of aging. Results indicate that these demographic models lead to mortality schedules that do not follow from the observed mortality record and that are inconsistent with predictions of biologically based limits to longevity. Although there is probably not a genetic program for death, the biology of our species places inherent limits on human longevity. As a human race, we were not built to last forever, but attention to some lifestyle habits may increase our chances of reaching the biological limit for our life span.2
Sex Differential in Longevity
There is a sex differential in longevity, with women outliving men. There may be biogenetic, environmental, and psychosocial perspectives that may be responsible for this sex differential3:
• Wellness and preventive health are practiced more by women than by men, and may explain some of the discrepancies. Women are more apt to go for Pap smears, mammograms, and cholesterol and BP checks than men are apt to go for cholesterol and BP checks.
• In general, men abuse their physical health more than women, and are more likely to indulge in alcohol and smoking.
• Women arguably have better coping mechanisms with respect to mental health as they may have better support and social systems.
• Women’s occupational role, by and large, focuses on family, and hence the stressor events are much different from those of men.
• Spiritual health in women on the average is better than in men, and hence their coping mechanisms are much better.
• Poor environmental health can decrease longevity, and in some men who are employed in vocations such as mining, fishing, the military, etc., life expectancy can be shortened.
• Last, but not least, the genetic makeup of men and women is different. Perhaps the Y chromosome puts a limit on longevity.
Over the years, there have been many theories put forth as to the longevity differential. Most of these theories have been based on the animal kingdom, but much can be learned. For instance, higher death rates among male animals have been linked to the more risky behaviors that males exhibit, including fighting each other over females. A Scottish study from the University of Stirling shows that male wild animals have more parasitic infections than do females at the time of death.4 The researchers studied parasites in all kinds of mammals and determined that males have significantly more infections. The researchers postulate that males are generally larger than females, thus providing larger targets for parasites and animals that carry them. Another reason may be testosterone, which can suppress the immune system, making the body more prone to infection. The accompanying editorial in Science states that the parasite explanation might apply to humans, too, because men are more susceptible to parasitic and infectious diseases than are women. The editorialists point out that men in the United States, the United Kingdom, and Japan are twice as vulnerable as women to parasite-induced death. And in Kazakhstan and Azerbaijan, where parasite-related death rates are high, men are more than four times more vulnerable than women.
In another theory, based on studying people who live 100 years, researchers conclude that menopause may be a major determinant of the life spans of both women and men. Women’s life span depends on the balance of two forces: the evolutionary drive to pass on one’s genes, and the need to stay healthy enough to rear as many children as possible. Menopause draws the line between the two. It protects older women from the risks of bearing children late in life, and lets them live long enough to take care of their children and grandchildren. It is interesting to note that most animals do not undergo menopause. It seems that menopause evolved in part as a response to the amount of time that the young remain dependent on adults to ensure their survival. Pilot whales, for example, suckle their young until the age of 14 years, and they, along with humans, are two of the few species that menstruate. The theory assumes that longevity is linked to a later menopause. Hence, there is the argument for hormone replacement therapy for postmenopausal women. This may extend perhaps to testosterone replacement in postandropausal men.
Interestingly, in their studies of centenarians, Perls and Fretts5 found that a surprising number of women who lived to be 100 or more gave birth in their 40s. These 100-year-old women were four times as likely to have given birth in their 40s as women born in the same year who died at age 73. A study of centenarians in Europe by the Max Planck Institute of Demography in Germany found the same relationship between longevity and fecundity. Factors that allow certain older women to bear children, including a slow rate of aging and decreased susceptibility to disease, improve a woman’s chances of living a long time. Extending that idea, it is possible that the driving force of human life span is maximizing the time during which woman can bear children. The age at which menopause eliminates the threat of female survival by ending further reproduction may therefore be the determinant of subsequent life span.
The menopause theory, however, does not fully explain why women live so much longer than men. In all developed countries and most undeveloped ones, women outlive men, sometimes by a margin of 10 years. The gender gap for the longevity differential is most pronounced in those who live 100 years or more. Among centenarians worldwide, women outnumber men nine to one. The mortality gap even varies during other stages of life. For example, between the ages of 15 and 24 years, men are four to five times more likely to die than women. This time frame coincides with the onset of puberty and an increase in reckless and violent behavior in males. Researchers sometimes refer to it as a “testosterone storm” as most deaths in this male group come from motor vehicle accidents, followed by homicide, suicide, cancer, and drowning. After the age of 24 years, the difference between male and female mortality narrows until late middle age. In the 55- to 64-year-old range, more men than women die, due mainly to heart disease, suicide, car accidents, and illnesses related to smoking and alcohol use. Heart disease kills 5 of every 1000 men in this age group.
It does seem that women have been outliving men for centuries and perhaps longer. Even with the sizable risk conferred by childbirth, women have outlived men since at least the 16th century. It is interesting to note that, in the United States between 1900 and the 1930s, the death risk for women of childbearing age was as high as that for men. Since then, improved health care, particularly in childbirth, has put women ahead of men again in the survival struggle, as well as raising life expectancy for both sexes. Sadly, almost like an equalizer, longevity doesn’t equate with quality of life. Although men may die of fatal illnesses like heart disease, stroke, and cancer, women tend to live on with nonfatal conditions such as arthritis, osteoporosis, diabetes, and dementia.
One contributor to the gender difference in life span may be the influence of sex hormones, in particular estrogens. The impact of testosterone on longevity is yet to be established. Estrogen lowers low-density lipoprotein (LDL) cholesterol and raises high-density lipoprotein (HDL) cholesterol. Another theory of longevity is based on survival. The fittest live longer, and arguably the longer a woman lives, the more slowly she ages, and the more offspring she can produce and rear to adulthood. Therefore, evolution would naturally select the genes of such women over those who die young. Long-lived men would also have an evolutionary advantage. Studies of chimps, gorillas, and other species closely related to humans suggest that a male’s reproductive capacity is actually limited more by access to females than by life span. And because men have not been involved in child care as much as women, survival of a man’s offspring, and thus his genes, depended not so much on how long he lived, but on how long the mother of his children lived.
The hypothesis that the Y chromosome may be a limiter to life span could be challenged. The longevity differential can be closed with lifestyle modification. Whether the average person drinks and smokes, on the one hand, or exercises and eats vegetables, on the other, subtracts 5 to 10 years from one’s life or adds 5 to 10 years to one’s life. But to live an additional 30 years requires the kind of genes that slow down aging and reduce susceptibility to conditions such as Alzheimer’s disease, stroke, heart disease, and cancer. Clues about what those genes are and how they work could come from studying those who survive 100 years or more. The New England Centenarian Study is the only scientific investigation of the oldest people performed in the United States.6 Centenarians are a tremendous resource for the discovery of genes responsible for aging and the ways in which aging occurs. Discovering these genes could lead to testing people and determining who might be disposed to accelerated aging via diseases such as Alzheimer’s, cancer, heart disease, and stroke. Such individuals might eventually be treated to extend the prospect of their living longer.
Although women can expect to live longer than men, the gap is closing. Death rates have begun to converge in the past 20 years. Some researchers attribute the convergence to women taking on the behaviors and stresses formerly considered the domain of men, including smoking, drinking, and working outside the home. Death rates from lung cancer have almost tripled in women in the past 20 years. On average, middle-aged female smokers live no longer than male smokers.
Mood and Longevity
It has been assumed that people with better moods end up living longer. Depressed individuals, especially men, are more likely to take their own lives. In a recent study, however, it was found that mildly depressed older women tend to live longer than those who are not depressed at all.7 The findings are contrary to most other studies on the link between depression and mortality.
The finding that women with mild depression live longer suggests a survival mechanism. The Duke study7 was based on a group that started with 2401 women and 1269 men, all older than 65. They were interviewed about their health at roughly 3-year intervals from 1986 to 1997 and were separated into three categories: depressed, mildly depressed, and not depressed. Of the women, 10.5% were considered mildly depressed. The women with mild depression were, on average, 60% were less likely than other women to die during any 3-year period. Researchers took into account age, chronic illness, and other factors in calculating the mortality rate.
Interestingly, the researchers found that depression had no influence on the mortality of men. This study may support a theory that says mild depression may allow people to cope more easily with their problems and remove themselves from dangerous or harmful situations.
Summary of Existing Theories
There are several theories that explain longevity in different species.8 Most, if not all, of the theories are derived from animal models. As such, it is important for the clinician not to go overboard in recommending a particular strategy for life extension based on theory alone. However, these theories should be used in conjunction with conventional medical practice, and that would include lowering cardiovascular and cerebrovascular risk factors including blood pressure and cholesterol levels. These simple measures can realistically increase the life span. There is a lot of hype about life extension, but the clinician should stress lifestyle changes, exercise, and nutrition. It is important not to give false hope to patients or to rely on the “pseudoscience” of some anti-aging products.9
Gene Regulation Theory
This theory focuses on the genetic programming encoded within our DNA. We are born with a unique genetic code and a predetermined tendency to certain types of physical and mental functioning. That genetic inheritance has a great deal to say about how quickly we age and how long we live. Each of us has a biological clock ticking away, set to go off at a particular time, give or take a few years. When that clock goes off it signals our bodies first to age and then to die. However, this genetic clock is subject to enormous variations, depending on what happens to us as we grow and on how we actually live. This is essentially the nature versus nurture debate. The differential life span of Japanese in Hawaii versus those in Japan typifies this debate about whether nature or nurture is more important.
CLINICAL IMPLICATIONS
The life span seems to be the longest in certain areas of the world including Okinawa (Japan), Sardinia (Italy), Kerala (India), and Scandinavia. It could be the genetics of these groups of people that result in longevity. However, the nurture debate may confound the true longevity rates. For instance, Okinawans eat mainly seafood, which is rich in omega oils that may protect the heart. Sardinians likewise have a Mediterranean diet that includes seafood, olives, and red wine, which have antioxidant properties. Keralans have one the highest educational levels in India, and education could facilitate taking care of oneself. Scandinavians, on the other hand, have a good socialized health care system that provides good access to care. All these factors can contribute to longevity, other than genes alone.
It has been hypothesized that genes control longevity. In one such study, the frequencies of 80 human leukocyte antigen (HLA) phenotypes in 82 centenarians and 20 nonagenarians in Okinawa, Japan, were compared with those in other healthy adults in various age brackets.10 Subjects over age 90 had an extremely low frequency of HLA-DRw9 and an increased frequency of HLA-DR1. In this age group the corrected relative risk (for number of antigens) and the p value for HLA-DRw9 were 5.2 and .0001, respectively; those for HLA-DR1 were 13.3 and .0367, respectively. Because a high frequency of DRw9 and a low frequency of DR1 are associated with autoimmune or immune deficiency diseases, the genetic protection against these disorders may contribute to longevity. A review by Ivanova et al11 suggested links with other alleles as well, and this is demonstrated in Table 2-1.
DNA Repair Capability
In 1963, Dr. Leslie Orgel of the Salk Institute suggested that because the “machinery for making protein in cells is so essential,… an error in that machinery could be catastrophic to human survival.” The body’s DNA is so vital that natural repair processes kick in when an error occurs. However, the body’s system is unable to do this all the time, and thus accumulation of these flawed molecules can cause disease and other age changes to occur. If the DNA repair process did not exist, scientists estimate that enough damage would accumulate in cells in 1 year to make them nonfunctional. Others believe that aging is caused by DNA damage through intrinsic mutations or other age-related nonmutational or epigenetic changes such as altered methylation.
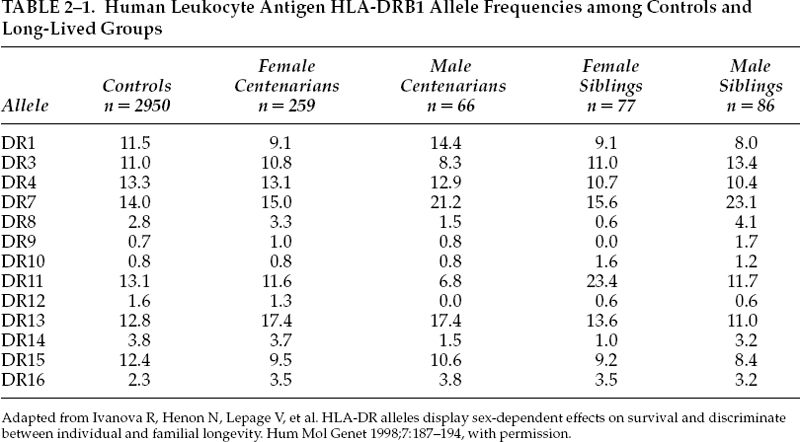
FIGURE 2-1. DNA status of different organs in young and old mice. (Adapted from Zahn RK. DNA status in brain and heart as prominent co-determinants for life span? Mech Ageing Dev 1996;89:79–94, with permission.)
In a laboratory experiment, Zahn12 demonstrated that the DNA repair capacities of young and old mice were indeed different (Fig. 2-1). Alkaline filter elution has been modified by a freeze-grinding step that allows the evaluation of the DNA status of whole tissue, including mouse tail cross sections, with only small additional artifacts. Four to seven different organs from individually coordinated female mice, rated as young as 2 to 3 months of age and as old as 24 to 27 months of age, have been used. Tissues of individual mice differ significantly in their DNA status. Alkali-labile sites are relatively rare and differ in amount in the different organs in the young. They show significant increases in the old, reaching the highest values in the brain and the heart. Proteinase K–dependent DNA–protein cross-links are not prominent, nor are they increased with age in some organs, except in the brain and the heart. DNA damage susceptibility was measured after application of 3.5 µM nitroquinoline-N-oxide to 15-mg fresh tissue pieces for 90 minutes. The susceptibility is large and varies in wide ranges in the different organs. Upon 3-hour postexposure incubation in full medium, all samples showed DNA repair; the young reach nearly complete repair in the lung, whereas repair in the old is generally significantly decreased. In old brain and heart it is even near zero. This together with high values in alkali-labile sites and DNA–protein cross–linking suggests that these two organs may act as pacemakers and play a role as prominent codeterminants for the life span of the species.
CLINICAL IMPLICATIONS
Some patients may be tempted to purchase and use nutraceuticals or cosmetics that claim to be able to restore vitality by “repairing DNA.” The clinician should tell the patient that although the theory sounds interesting, at least in fruit flies and mice, there is no evidence that these substances actually work in human beings.
Telomeres and Telomerase
Telomeres are bits of DNA on the ends of our chromosomes; the parallel would be the hard ends of shoelaces. Fig. 2-2 is a graphic representation of telomeres. Although they do not contain genes, telomeres are important for replication or duplication of the chromosomes during cell division.
Telomere length recently has been described as a marker of cellular aging. The originator of the theory is Olovnikov13 In 1973, he proposed that cells lose a small amount of DNA following each round of replication due to the inability of DNA polymerase to fully replicate chromosome ends (telomeres) and that eventually a critical deletion causes cell death. Telemetries are specialized DNA sequences located at the end of eukaryotic chromosomes. In humans, telomeres are composed of repeats of the sequence TTAGGG reiterated in tandem for up to 15 kilobases at birth. Telomeres are synthesized by telomerase, a ribonucleoprotein reverse transcriptase enzyme that maintains the lengths of chromosomes. Loss of telomeres can lead to DNA damage. This association of telomere shortening and senescence in vitro has been established. Cells that have been supplied with an exogenous source of telomerase maintain a youthful state and proliferate indefinitely.
FIGURE 2-2. Graphic representation of telomeres, located at end of chromosomes.
CLINICAL IMPLICATIONS
It is a notion that in normal human organs with a capacity for cell replacement, the telomere clock may allow enough divisions for normal growth, repair, and maintenance. However, this may not be enough to enable additional cell replications needed during chronic disease. A potential remedy may be found by increasing the life span of tissue cells, by telomerase. Another possibility may involve taking cells from an individual, extending the life span of the cells in vitro by telomerase, and then reintroducing the cells into the organ that requires help. At this point, these possibilities are still experimental, and genetic engineering may prove useful for some diseases in the future, and perhaps for extending life.
Endocrine Theory of Aging
Aging is followed by a fall in neuroendocrine functions, resulting in a decreased secretion of sex steroids and growth hormone. When we are young, hormone levels tend to be highest. This accounts for, among other things, menstruation in women and high libido in both sexes. As we age there is a decline in the function of the hypothalamic-pituitary adrenal axis. This results in age-related changes. For example, decline in growth hormone may result in loss of muscle mass. Drops in levels of testosterone and thyroxine can increase the fat-to-muscle ratio.
With aging, cortisol may be inadequately secreted upon stress challenges. This could be due to deficient functioning of central glucocorticoid receptors. In combination, these endocrine perturbations probably result in changes in psychological factors such as energy and well-being, altered body composition, and insulin resistance, as well as other risk factors for diseases characteristic of the aging man.
CLINICAL IMPLICATIONS
Hormones such as estrogens are frequently prescribed for women in the postmenopausal state. The Women’s Health Initiative’s recently published study had raised some doubt about the use of an estrogen/progesterone combination.14 Increasingly, testosterone is prescribed for men as they age. Preventive and therapeutic interventions with hormones can indeed improve quality of life. Whether hormone replacement results in longevity is yet to be determined.
Free Radical Theory of Aging
Dr. R. Gerschman first introduced this theory of aging in 1954, and later Dr. Denham Harman of the University of Nebraska College of Medicine further developed this theory. Free radical is a term used to describe any molecule that differs from conventional molecules in that it possesses a free electron. This is a property that makes molecules react with other molecules in highly volatile and destructive ways. They can attack cell membranes, creating metabolic waste products, including substances known as lipofuscins. An excess of lipofuscins in the body is shown as darkening of the skin in certain areas, so-called aging spots. Lipofuscins, in turn, interfere with the cells’ ability to repair and reproduce themselves. They disturb DNA and RNA synthesis of protein, lower our energy levels, prevent the body from building muscle mass, and destroy cellular enzymes, which are needed for vital chemical processes. Substances that prevent the harmful affects of free radicals are called antioxidants. Natural antioxidants include vitamin C, E, and A. As shown in Fig. 2-3, lipid peroxidation and changes in status of antioxidant compounds such as glutathione and others could be measures of oxidative stress.
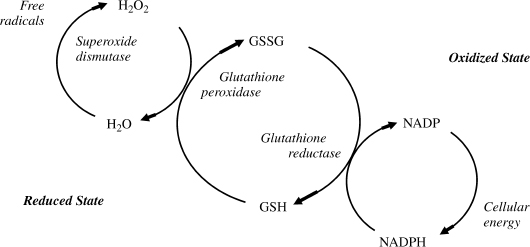
FIGURE 2-3. Besides lipid peroxidation, changes in status of antioxidant compounds such as glutathione and others could be measures of oxidative stress. GSSG, oxidized glutathione; GSH, reduced glutathione; NADP, nicotinamide adenine dinucleotide phosphate; NADPH, reduced NADP.
CLINICAL IMPLICATIONS
Most commonly, measurements of oxidative stress are by-products of lipid peroxidation, but changes in status of antioxidant compounds such as glutathione, protein and DNA oxidation products, and antioxidant enzyme activities have also been used. These are all indirect measures of free radical activity. Electron spin resonance, a direct measure of free radicals, has been used predominantly in in vitro studies, but it recently has been used to detect free radicals in blood.
In addition, many patients take vitamins as antioxidants. Some small clinical trials suggest that patients taking vitamins like E and C are less likely to develop illnesses. Even if antioxidants could provide the benefits suggested by epidemiology studies, smoking cessation and other lifestyle factors would have a far greater effect on the rates of lung cancer and coronary heart disease. Overall, the benefits of taking high doses of vitamin E remain to be established. At present, there is no convincing evidence that taking supplements of vitamin C prevents any disease.
Low Calorie Theory of Aging
In the 1930s, researchers discovered that they could extend the life of rats by 33% if they limited them to a very low-calorie diet. Oxidative stress is a major factor in aging and cellular senescence. It has been theorized that dietary restriction without malnutrition protected rats and mice against oxidative stress, decreased oxidative damage, and decreased accumulation of oxidatively damaged proteins within the mitochondria. The animals lived longer, suffered fewer late-life diseases, and appeared more youthful, and their bodies’ biological aging processes were slowed.15 Since that time, scientists have produced similar life-extending results with many other creatures, ranging from fruit flies to fish.
Is caloric restriction per se responsible for the observed benefits, or is some other factor that is reduced when calories are restricted? Studies show that limiting fat, protein, or carbohydrate, without accompanying caloric reduction, does not seem to increase maximum life span. Nor does supplementation with extra antioxidants and multivitamins. Varying the types of fats, carbohydrates, and proteins ingested also had no effect. In fact, no other intervention except caloric restriction has yet been shown to slow aging.
At this point, it is unclear that human life spans can be increased with calorie restrictions. Humans live much longer than many other creatures, and thus such studies are difficult to do. Studies with primates provide some clues. Investigations on monkeys have been underway since 1987, and preliminary results suggest that caloric restriction increases both health and life span in primates. Biomarkers of aging, such as insulin levels, glucose levels, and blood pressure, have led researchers to conclude that monkeys eating less age more slowly. In most such studies, calories are restricted to 30 to 50% of what the animal would normally eat. Care is taken to see that enough vitamins, minerals, protein, and fat are ingested for the proper functioning of tissues. The participants of the Biosphere-2 experiment in Arizona were forced to eat a low-calorie diet for 2 years because their food production was less than projected. They experienced the same anti-aging trends in biomarkers as were found in the monkey experiments. Fig. 2-4 shows blood pressure changes in the Biosphere-2 participants on this diet.
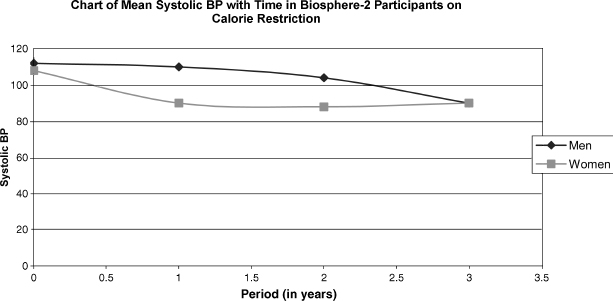
FIGURE 2-4. Human participants of Biosphere-2 demonstrating lowering of systolic BP with calorie restriction.
CLINICAL IMPLICATIONS
The clinician is often asked to advise on weight loss by means of a low-calorie diet. A low-calorie diet is one that is below 1000 calories per day. Often, the aim of a low-calorie diet is to lose weight so as to lower blood pressure and cholesterol levels and to ameliorate arthritis. Most people do not realize that a low-calorie diet, with the appropriate nutrients, may actually lead to a longer and better life. Patients may not only have better health outcomes, but they also feel better as well. Losing weight is one of the best anti-aging antidotes. Patients who actively lose weight often do better in the long run than those who rely just on medications or nutraceuticals.
Immunologic Theory of Aging
The thymus may be the master gland of the immune system. The size of this gland shrinks from 250 g at birth to around 3 g by age 60. The reduction in size and function of the thymus is associated with senescence. Some studies have shown that thymic factors are helpful in restoring the immune system of children born without them as well as rejuvenating the poorly functioning immune system of the elderly. Dr. R.L. Walford first proposed the immunologic theory of aging. Thymic hormones may also play a role in stimulating and controlling the production of neurotransmitters and brain and endocrine system hormones, which implies that they may be the pacemakers of aging itself besides being key regulators responsible for immunity.
CLINICAL IMPLICATIONS
Some health stores sell thymic extracts, suggesting that their intake may lead to life extension. Thymic extracts are essentially protein, and they get broken down in the digestive tract and absorbed as amino acids. They are not proven to be useful clinically in extending life or fighting infections. However, it is well recognized that poor immunity leads to illness, morbidity, and mortality.
Discussion of Case History
Dr. Terror, introduced at the start of this chapter, requested to be on testosterone replacement therapy. He also inquired about growth hormone replacement therapy, but changed his mind after finding out the cost of the treatment. He wondered about the advertised growth hormone secretagogues, but was advised that they rarely work. Quite a bit of time was spent discussing diet and exercise with him. As he was fairly thin, strength-training exercises were suggested for him. He decided to join a gym and exercised 45 minutes three to five times a week. He increased protein intake, fresh vegetables, and fruits (potential antioxidants). He was prescribed testosterone gel 5mg/day. After 6 months, on a routine follow-up visit, he was very satisfied. Overall, he said, his mood is better, and he is sharper and more energetic. He said the program restored some of his lost years. Objectively, it was noticed that despite weighing the same at 150 pounds, his body fat was reduced to 19%, implying muscle growth. His skin was less dry, his libido better, and he thought that testosterone was the elixir of youth for him. He was advised that testosterone was only supplementary and that it was the combination of testosterone with exercise and the right nutrition that seemed to have made him feel five years younger.
Conclusion and Key Points
Men on average live about 7 or 8 years less than women. Genetics and lifestyle account for the difference. Paying attention to simple issues like weight, smoking cessation, nutrition, and exercise can alter longevity in men. The role of hormone replacement, at least in the short term, has been shown to improve the quality of life of not only women but also of men. The keys to longevity can be summarized in the following few points:
• Lifestyle habits from youth, if altered in midlife, can alter outcomes in later life.
• Modest food intake, antioxidants, hormones, and exercise can alter the outcome for some individuals.
• Positive mood, being married, and not smoking are predictors of longevity.
• There are many theories of aging. Care must be taken in interpreting those that are based on animal studies. The human body is more complex, and applying some of these theories to humans can be erroneous.
• The best antidotes for aging are weight loss through proper nutrition, exercise, smoking cessation, alcohol moderation, and a positive outlook.
REFERENCES
1. Harman D. Aging: phenomena and theories. Ann NY Acad Sci 1998;854:1–7
2. Olshansky SJ, Carnes BA, Desesquelles A. Demography. Prospects for human longevity. Science 2001;291:1491–1492
3. Waldron I. Causes of sex differential in longevity. J Am Geriatr Soc 1987;35:365–366
4. Moore SL, Wilson K. Parasites as a viability cost of sexual selection in natural populations of mammals. Science 2002; 297: 2015–2018
5. Perls TT, Fretts RC. The evolution of menopause and human life span. Ann Hum Biol 2001;28:237–245
6. Hitt R, Young-Xu Y, Silver M, Perls T. Centenarians: the older you get, the healthier you have been. Lancet 1999;354: 652
7. Hybels CF, Pieper CF, Blazer DG. Sex differences in the relationship between subthreshold depression and mortality in a community sample of older adults. Am J Geriatr Psychiatry 2002;10:283–291
8. Masoro EJ, Austad SH. In: Handbook of the Biology of Aging. San Diego: Academic Press; 2001
9. Olshansky SJ, Hayflick L, Carnes BA. Position statement on human aging. J Gerontol A Biol Sci Med Sci 2002;57: 292–297
10. Takata H, Suzuki M, Ishii T, Sekiguchi S, Iri H. Influence of major histocompatibility complex region genes on human longevity among Okinawan-Japanese centenarians and nonagenarians. Lancet 1987;10:824–826
11. Ivanova R, Henon N, Lepage V, et al. HLA-DR alleles display sex-dependent effects on survival and discriminate between individual and familial longevity. Hum Mol Genet 1998;7: 187–194
12. Zahn RK. DNA status in brain and heart as prominent co-determinants for life span? Mech Ageing Dev 1996;89:79–94
13. Olovnikov AM. Telomeres, telomerase, and aging: origin of theory. Exp Gerontol 1996;31:443–448
14. Rossuw JE, Andersen GL, Prentice RL, et al. Risks and benefits of estrogens and progestin in healthy postmenopausal women. JAMA 2002;288:321–323
15. Kolestky S, Puterman DI. Effect of low calorie diet on hyperlipidemia, hypertension and life span of genetically obese rats. Proc Soc Exp Biol Med 1976;151:368–371
< div class='tao-gold-member'>









