Li-Li Tong, Sharon Adler
Prevention and Treatment of Diabetic Nephropathy
In diabetic patients, the development of diabetic nephropathy (DN) signifies the presence of a generalized microvascular syndrome that is frequently accompanied by macrovascular disease (see Chapter 30). Classically, DN evolves through several clinical stages based on urine albumin excretion (UAE) values: normoalbuminuria, microalbuminuria, and macroalbuminuria, or overt nephropathy. Regression of microalbuminuria to normoalbuminuria occurs spontaneously in a substantial proportion of patients with type 1 and type 2 diabetes. Without treatment, however, patients with persistent microalbuminuria are at high risk of progressing to overt nephropathy.
There is consensus that the level of UAE has predictive importance for both renal outcome and cardiovascular (CV) morbidity and mortality and affects the choice of therapeutic intervention. In patients with established DN, regression to normoalbuminuria and preservation of renal function, although difficult, is the ideal treatment goal. Strict blood pressure (BP) and glycemic control early in the disease course is vital. Aggressive lipid-lowering and lifestyle modifications, including adherence to low-protein and low-sodium diet, exercise, weight loss, and smoking cessation all are beneficial and likely to improve renal and CV outcome. Most patients with advanced-stage diabetic kidney disease, however, are likely to progress relentlessly to end-stage renal disease (ESRD), even though treatment may slow this progression.
This chapter reviews the current preventive and therapeutic strategies that promote renoprotection and cardioprotection in diabetic patients (Fig. 31-1). In general, the treatment principles for established DN are similar to those adopted for the prevention of DN, although multiple and more intensive strategies may be required for treatment. Special considerations are indicated in the management of the diabetic patient with advanced chronic kidney disease (CKD) (see Chapter 32). Many therapeutic issues discussed here are not specific for DN and thus are also relevant for CKD in general (see Chapter 80).
Prevention of Diabetic Nephropathy
Prevention and early detection of DN improve patient outcome. General measures for prevention of DN include glycemic control and rigorous BP control. Because diabetes is associated with increased risk for CV morbidity and mortality, treatment of dyslipidemia as well as diet and lifestyle modifications, including physical activity and weight reduction as appropriate and smoking cessation, can significantly lower the CV risks.
Glycemic Control
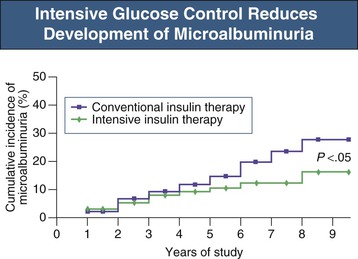
In type 1 diabetic patients, strict glycemic control decreases the risk for microalbuminuria and impaired glomerular filtration rate (GFR). The Diabetes Control and Complications Trial (DCCT) compared the effects of intensive glucose control with conventional treatment on the development and progression of the long-term complications of type 1 diabetes (Fig. 31-2). During a 9-year period, patients receiving intensive therapy (mean hemoglobin A1c 7%) had a 35% to 45% lower risk for development of microalbuminuria compared with the control group (mean HbA1c 9%).1 More recently, DCCT and Epidemiology of Diabetes Interventions and Complications (EDIC) trial data indicated that the long-term risk of an impaired GFR was lower by 50% in patients treated for an average of 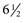 years with DCCT intensive glucose control than among those treated with conventional therapy. This effect was not evident until more than 10 years after randomization, beyond the period of the DCCT treatment intervention.2 Moreover, the restoration of euglycemia with pancreas transplantation prevents recurrent DN in renal allograft of type 1 diabetic patients.3
years with DCCT intensive glucose control than among those treated with conventional therapy. This effect was not evident until more than 10 years after randomization, beyond the period of the DCCT treatment intervention.2 Moreover, the restoration of euglycemia with pancreas transplantation prevents recurrent DN in renal allograft of type 1 diabetic patients.3
For type 2 diabetic patients, several major studies have demonstrated a lower risk of nephropathy with stricter glycemic control. In a study design similar to the DCCT, the Kumamoto study found a 60% reduction in microalbuminuria in relatively young, nonobese type 2 diabetic patients receiving intensive glycemic treatment (HbA1c 7.1%) compared with conventional treatment (HbA1c 9.4%).4 In the United Kingdom Prospective Diabetes Study (UKPDS) trial, newly diagnosed patients with type 2 diabetes were randomly assigned to intensive management (HbA1c 7.0%) with a sulfonylurea or insulin or to conventional management (HbA1c 7.9%) with diet alone. After 9 years of intensive therapy, relative risk reduction for the development of microalbuminuria was 24%.5 After termination of the study, patients were observed for a further 10 years. The differences in HbA1c were lost within 1 year, but a 24% lower risk of microvascular disease and myocardial infarction (−15%) persisted. All-cause mortality (−13%) was also reduced. This phenomenon of ongoing beneficial effects on diabetic complications after a period of improved glycemic control even if followed by a return to less intensive metabolic control has been described as representing “metabolic memory” by the DCCT/EDIC investigators and as a “legacy effect” by the UKPDS investigators. This observation underlines the importance of early glycemic control before complications develop.
Three recent major trials testing whether glycemic control reduced cardiovascular disease (CVD) risk in type 2 diabetic patients have refined our approach to establishing glycemic goals. The Action in Diabetes and Vascular Disease, Perindopril and Indapamide Controlled Evaluation (ADVANCE) study showed that intensive blood glucose control (HbA1c 6.5% vs. 7.3%) yielded a 10% relative reduction in the combined outcome of major macrovascular and microvascular events, primarily because of a 21% relative reduction in nephropathy.6 However, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, very tight glycemic control (lowering of HbA1c to median of 6.4% vs. 7.5% with conventional control) was associated with 22% increase in mortality from any cause and did not significantly reduce major CV events.7 A third major study of tight glucose control in type 2 diabetics, the Veterans Affairs Diabetes Trial (VADT), found no significant reduction in CV deaths or events during 7.5 years in high-risk patients treated aggressively for glycemic control (median HbA1c 6.9%) compared with standard therapy (median HbA1c 8.4%).8
It is apparent that glycemic control in type 2 diabetic patients must be individualized and take into account the patient’s age, duration of diabetes, presence of CVD, presence of CKD, and microvascular risks and complications, as well as previous glycemic control and susceptibility to and awareness of hypoglycemia. In younger patients with recently diagnosed diabetes and no prior CVD events, strict glycemic control can reduce the risk of nephropathy and other microvascular complications. In patients with longstanding diabetes and known CVD, data do not support glycemic control to HbA1c of less than 7% in reducing the risk of further CVD events or mortality. The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) guideline recommends lowering of HbA1c levels to 7.0% for both type 1 and type 2 diabetic patients.9 At even lower glycemic targets, any potential renoprotective effects of tight glucose control must be counterbalanced by the possibility of more frequent hypoglycemic episodes.
Blood Pressure Control
In patients with type 1 diabetes, microalbuminuria typically precedes hypertension. In patients with type 2 diabetes, however, as many as 40% are hypertensive before the diagnosis.10 In both type 1 and type 2 diabetic patients, higher BP is associated with increasing albuminuria and with more rapid progression and increased risk of kidney failure.11 Early treatment of hypertension is critical for prevention of DN, retinopathy, and CVD. Major guidelines published before the Action to Control Cardiovascular Risk in Diabetes Blood Pressure (ACCORD BP) trial have suggested that the target blood pressure in diabetic patients should be less than 130/80 mm Hg.12 However, this BP target was challenged by findings of the ACCORD BP trial. Among diabetic patients with high CV risk randomized to goal systolic BP of less than 120 mm Hg or standard therapy aiming for under 140 mm Hg, there was no difference in the risks of composite major CV events.13 A cross-sectional analysis of patients in the Swedish National Diabetes Registry also failed to show a reduction in mortality in patients with systolic BP below 130 vs. 130 to 139 mm Hg.14 The recent Kidney Disease: Improving Global Outcomes (KDIGO) guideline for diabetic patients with CKD recommends a target BP of 140/90 mm Hg or less for all diabetic patients, and 130/80 mm Hg or less for patients with urine albumin excretion of greater than 30 mg per 24 hours.15 For diabetic patients at highest risk for cerebrovascular accident (CVA, stroke), even lower systolic BP goals may provide greater protection against stroke, but the potential risks and burdens of serious adverse events attributable to antihypertensive therapy must be considered with such treatment goals.
Renin-Angiotensin System Blockade in Prevention
The role of blockade of the renin-angiotensin system (RAS) in normotensive, normoalbuminuric diabetic patients for the primary prevention of DN is unproved and cannot be recommended at this time. Most patients with diabetes do not develop DN, even after long periods of uncontrolled hyperglycemia, and there are hazards with the use of RAS-blocking drugs, including their potential teratogenicity in pregnancy. In a post hoc analysis of three randomized controlled trials (RCTs) conducted as part of the multicenter Diabetic Retinopathy Candesartan Trials (DIRECT) program, which included normotensive and normoalbuminuric type 1 diabetics and normoalbuminuric type 2 diabetics with or without hypertension, the ARB candesartan was found to have no effect on the development of microalbuminuria.16A recent study investigating whether olmesartan would prevent microalbuminuria in type 2 diabetic patients showed that the incidence of microalbuminuria was slightly reduced from 9.8% to 8.2% in the olmesartan arm, but this difference disappeared after adjusting for the lower BP in that arm. Of potential concern, was a higher rate of fatal CV events with this ARB among patients known to have preexisting coronary heart disease, especially those with lower BP.17
In hypertensive diabetic patients, an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) is effective as a first-line antihypertensive agent. The Bergamo Nephrologic Diabetes Complications Trial (BENEDICT), which randomized hypertensive normoalbuminuric type 2 diabetic patients to placebo, verapamil, trandolapril, or a combination of verapamil plus trandolapril, showed less progression to microalbuminuria in patients receiving trandolapril either alone or with verapamil.18 Verapamil alone was no different from placebo. There were similar findings in smaller studies with other RAS inhibitors, implicating a class effect.19 Longer-term studies would be required to demonstrate the effects of RAS blockade on the clinically important outcomes of death, dialysis, and doubling of serum creatinine level in normoalbuminuric patients. Therapy for the prevention of DN likely will be guided exclusively by studies using albuminuria as a surrogate.
Treatment of Dyslipidemia
Few clinical data are available on the effects of lipid lowering alone in preventing DN. In the Diabetes Atherosclerosis Intervention Study (DAIS), type 2 diabetic patients taking fenofibrate had a significantly lower rate of progression from normoalbuminuria to microalbuminuria at 3 years compared with the placebo group.20 Earlier clinical practice guidelines had emphasized specific treatment targets for low-density lipoprotein (LDL) cholesterol of below 100 mg/dl (2.6 mmol/l) for diabetic patients in general and below 70 mg/dl (1.8 mmol/l) for diabetic patients with CVD. These targets, however, have not been proven beneficial in any clinical trial. Newer guidelines from the American College of Cardiology and the American Heart Association are shifting away from specific LDL cholesterol treatment targets.21 Emphasis is now placed on assessing a patient’s global risk for CVD and using maximum tolerated statin intensity for primary and secondary prevention of CVD.
Nonpharmacologic Interventions
For all diabetic patients, emphasis should be placed on lifestyle modification to lower the risk of diabetic kidney disease and CV events, including dietary restriction of salt and saturated fat, weight reduction and exercise as appropriate, and smoking cessation. Smoking in particular is an independent risk factor for the development of nephropathy in type 2 diabetes and is associated with an accelerated loss of renal function.22
Treatment of Diabetic Patients with Microalbuminuria or Overt Nephropathy
For diabetic patients with incipient or established DN, the optimal therapeutic approach to reduce the rate of progression of nephropathy and to minimize the risk for CV events involves aggressive management of hypertension with emphasis on a RAS blocker, combined with management of dyslipidemia, hyperglycemia, and albuminuria, as well as diet modification, exercise, and smoking cessation. Such multifactorial therapy in the Steno type 2 trial in type 2 diabetics included management of hyperglycemia and of hypertension, ACE inhibition, statins, aspirin, reduction of fat intake, light to moderate exercise, and cessation of smoking.23,24 Impressive lowering of the risk of CVD, nephropathy, retinopathy, and autonomic polyneuropathy was noted after 7.7 years, and even a delayed reduction of mortality was seen.
In general, patients with DN require multiple antihypertensive agents (including RAS-blocking agents) to achieve BP goal, intensive insulin therapy in type 1 diabetes, two or more drugs for glucose control in type 2 diabetes, at least one lipid-lowering agent, and an aspirin or other antiplatelet agent for CV protection. One obstacle to achieving adherence is the number of medicines and the complexity of these regimens. Therefore, treatment of patients with DN needs to be individualized and requires considerations of the cost, side effects, and convenience of the drug regimen. Regular monitoring of UAE and serum creatinine concentration to assess response to therapy and progression of disease is required.
Antihypertensive Treatment
In type 1 and type 2 diabetic patients with overt nephropathy, hypertension is an almost universal finding and is associated with volume expansion and salt sensitivity. The absence of hypertension in an untreated patient with overt nephropathy should raise suspicion for underlying cardiac problems. Uncontrolled hypertension is associated with more rapid progression of DN25 and increased risk of fatal and nonfatal CV events.26 Thus, effective treatment of systemic hypertension is arguably the single most important strategy in the treatment of established DN (Fig. 31-3). Some have suggested that the overall effect of BP lowering may be more important than the type of antihypertensive used.27 Antihypertensive therapies, regardless of agent used, reduce UAE, delay progression of nephropathy, postpone renal impairment, and improve survival in both type 1 and type 2 diabetic patients with DN.28
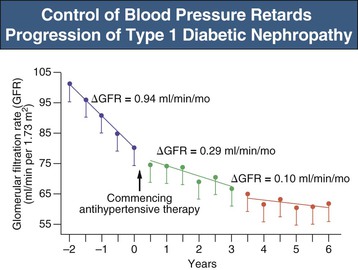
The optimal lower limit for BP control in DN though remains unclear. In a secondary analysis of the Irbesartan Diabetic Nephropathy Trial (IDNT), progressive lowering of systolic BP to 120 mm Hg was associated with improved renal and patient survival, an effect independent of baseline renal function.29 However, mortality increased with systolic BP below 120 mm Hg, although a cause-and-effect relationship cannot be inferred from the data. From a safety perspective, diastolic pressure is also important. Low diastolic BP is poorly tolerated, and the incidence of myocardial infarction and mortality increases at values below 70 mm Hg, at least in patients with coronary heart disease, presumably because coronary perfusion occurs only during diastole. Indeed, in the IDNT study, CV mortality increased not only with higher systolic pressure but also with low diastolic pressure. The recent ACCORD BP trial failed to show reduced CV events, but found increased rates of hyperkalemia and renal dysfunction, when targeting a systolic BP less than 120 mm Hg compared with less than 140 mm Hg.13 Thus, given the lack of strong evidence of benefit from reducing systolic BP to below 130 versus below 140 mm Hg, it is reasonable to strive for a BP target of 140/90 mm Hg or less for all diabetic patients. These recommendations are consistent with the KDIGO guideline and the Eighth Joint National Committee (JNC VIII) on BP management in diabetic patients.15
Renin-Angiotensin System Blockade in Treatment
In diabetic patients with established DN, RAS blockade with ACE inhibitors or ARBs confers preferential renoprotection that is independent of BP reduction. Intraglomerular hemodynamic and nonhemodynamic renal effects of angiotensin II (Ang II) best explain the observed renoprotection. Supporting this hypothesis, evidence-based in vitro models of DN show cellular effects of RAS inhibition that are consistent with benefit independent of BP effects (see Chapter 30).
Although many demonstrated a beneficial effect of ACE inhibitors and ARBs in retarding progressive renal disease, these studies did not differentiate between the relative contributions of the RAS blockade versus aldosterone system blockade. The renin-angiotensin-aldosterone system (RAAS) represents a hormonal cascade that functions in the homeostatic control of arterial pressure, tissue perfusion, and extracellular fluid homeostasis (see Chapter 7). In fact, plasma aldosterone levels are elevated in a subset of patients despite ACE inhibitor and ARB therapy (also known as aldosterone breakthrough; see Chapter 82). In studies that defined aldosterone breakthrough as any increase from an individual’s baseline serum aldosterone level (i.e., before ACE inhibitor or ARB therapy), the incidence ranged from 40% during 10 months to 53% during 12 months.30 In addition to its classic effects of promoting sodium retention and enhancing potassium and magnesium excretion, aldosterone promotes tissue inflammation and fibrosis.31 Small studies have demonstrated considerably faster decline in glomerular filtration rate (GFR) in patients who experienced aldosterone breakthrough (median 5.0 ml/min/yr) than in those who did not (median 2.4 ml/min/yr). Aldosterone blockade, independent of RAS blockade, reduces proteinuria and retards progression of nephropathy. Current evidence is not strong enough to support widespread screening for aldosterone breakthrough. However, in select patients, additional aldosterone blockade with close monitoring of serum potassium levels may represent optimal therapy for patients who show aldosterone breakthrough during treatment with an ACE inhibitor or an ARB and who no longer show maximal antiproteinuric effects with these agents.
Type 1 Diabetic Patients
In type 1 diabetic patients with microalbuminuria, ACE inhibitors reduce the risk of progression to overt nephropathy.32,33 In a meta-analysis of 12 placebo-controlled trials in 698 normotensive patients with type 1 diabetes and microalbuminuria treated with ACE inhibitors, the majority for more than 2 years, treatment was associated with a 60% reduction in progression to macroalbuminuria and a threefold increase in regression to normoalbuminuria.34
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree