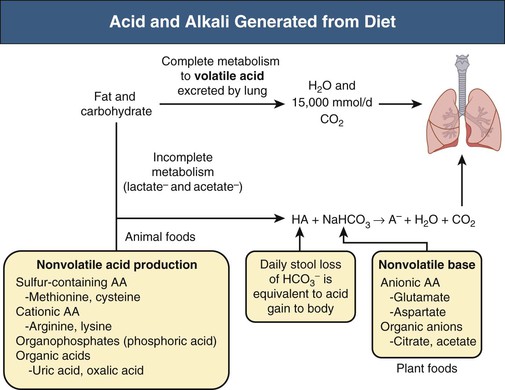
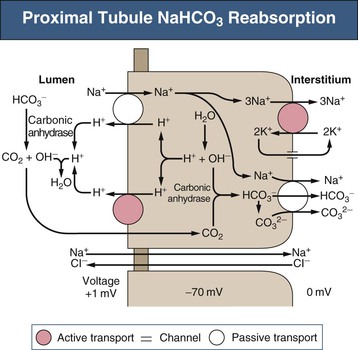
Biff F. Palmer The acid-base status of the body is carefully regulated to maintain the arterial pH between 7.35 and 7.45 and the intracellular pH between 7.0 and 7.3. This regulation occurs in the setting of continuous production of acidic metabolites and is accomplished by intracellular and extracellular buffering processes in conjunction with respiratory and renal regulatory mechanisms. This chapter reviews the normal physiology of acid-base homeostasis. Both acid and alkali are generated from the diet. Lipid and carbohydrate metabolism results in production of carbon dioxide (CO2), a volatile acid, at the rate of approximately 15,000 mmol/day. Protein metabolism yields amino acids, which can be metabolized to form nonvolatile acid and alkali. Amino acids such as lysine and arginine yield acid on metabolism, whereas the amino acids glutamate and aspartate as well as organic anions such as acetate and citrate generate alkali. Sulfur-containing amino acids (methionine, cysteine) are metabolized to sulfuric acid (H2SO4), and organophosphates are metabolized to phosphoric acid (H3PO4). In general, animal foods are high in proteins and organophosphates, thereby providing a net acid diet; plant foods are higher in organic anions and provide a net alkaline load. In addition to acid and alkali generated from the diet, there is a small daily production of organic acids, including acetic acid, lactic acid, and pyruvic acid. Also, a small amount of acid is generated by the excretion of alkali into the stool. Under normal circumstances, daily net nonvolatile acid production is approximately 1 millimole (mmol) of hydrogen ions (H+) per kilogram (kg) of body weight (Fig. 11-1). Intracellular and extracellular buffer systems minimize the change in pH during the addition of acid or base equivalents but do not remove acid or alkali from the body. The most important buffer system is that of the bicarbonate ion and carbon dioxide (HCO3−-CO2). In this system, carbon dioxide concentration, [CO2], is maintained at a constant level set by respiratory control. Addition of acid (HA) leads to conversion of HCO3− to CO2 according to the reaction HA + NaHCO3 → NaA + H2O + CO2. HCO3− is consumed, but [CO2] does not change because this is maintained by respiration. The net result is that the acid load has been buffered and pH changes are minimal. Whereas the HCO3−-CO2 buffer system is the most important of the buffers in extracellular fluid (ECF), other buffers such as plasma proteins and phosphate ions also participate in the maintenance of a stable pH. During metabolic acidosis, the skeleton becomes a major buffer source as acid-induced dissolution of bone apatite releases alkaline Ca2+ salts and HCO3− into the ECF. With chronic metabolic acidosis, this can result in osteomalacia and osteoporosis. The calcium released can result in hypercalciuria and an increased likelihood of renal stones. Within the intracellular fluid (ICF) compartment, pH is maintained by intracellular buffers such as hemoglobin, cellular proteins, organophosphate complexes, and HCO3− as well as by the H+-HCO3− mechanisms that transport acid and alkali in and out of the cell. Removal of acid or alkali from the body is accomplished by the lungs and kidneys. The lungs regulate the CO2 tension (Pco2), and the kidneys regulate the serum bicarbonate concentration, [HCO3−]. Although the HCO3−-CO2 buffer system is not the only buffer system, all extracellular buffer systems are in equilibrium. Because serum [HCO3−] is much greater than that of other buffers, changes in the HCO3−-CO2 buffer pair can easily titrate other buffer systems and thus set pH. The following Henderson-Hasselbalch equation explains how the lungs and kidneys function in concert: As can be seen, pH is determined by the ratio of HCO3− to CO2. Conditions associated with similar fractional changes in [HCO3−] and [CO2], such as when both are halved, will not change blood pH. The lungs defend pH by altering alveolar ventilation, which alters the CO2 excretion rate and thereby controls the arterial CO2 tension (Paco2) of body fluids. Systemic acidosis stimulates the respiratory center, resulting in increased respiratory drive that lowers the Paco2. As a result, the fall in blood pH is less than would have occurred in the absence of respiratory compensation. If the fractional change in Pco2 were similar to that in serum [HCO3−], blood pH would not change. However, respiratory compensation rarely normalizes blood pH, and thus the fractional change in Pco2 is less than the change in serum [HCO3−]. Quantitatively, the normal respiratory response in metabolic acidosis is a 1.2 mm Hg decrease in Paco2 for every 1 mmol/l decrease in HCO3−; the increase in Paco2 in response to metabolic alkalosis averages 0.7 mm Hg for every 1 mmol/l increase in HCO3− above baseline.1 Buffer systems and respiratory excretion of CO2 help maintain normal acid-base balance, but the kidneys provide a critical role in acid-base homeostasis. The kidneys normally generate sufficient net acid excretion (NAE) to balance nonvolatile acid produced from normal metabolism. NAE has three components, titratable acids, ammonium (NH4+), and bicarbonate, and is calculated by the following formula: where UAmV is the rate of NH4+ excretion, UTAV is the rate of titratable acid excretion, and UHCO3−V is the rate of HCO3− excretion. Under basal conditions, approximately 40% of NAE is in the form of titratable acids and 60% is in the form of ammonia (NH3); urinary bicarbonate concentrations and excretion are essentially zero under normal conditions. When acid production increases, the increase in acid excretion is almost entirely caused by an increase in excretion of NH4+. The glomerulus is not normally considered as participating in acid-base regulation. However, the glomerulus filters an amount of HCO3− equivalent to serum [HCO3−] multiplied by the glomerular filtration rate (GFR). Under normal circumstances, the filtered load of HCO3− averages approximately 4000 mmol/day. Normal acid-base homeostasis requires both the reabsorption of this filtered bicarbonate and the generation of “new” bicarbonate; the latter replenishes bicarbonate and other alkaline buffers consumed in the process of titrating endogenous acid production. From the standpoint of prevention of or correction of acidosis, GFR is not regulated by alterations in acid or base and therefore does not contribute to acid-base homeostasis. The proximal tubule reabsorbs approximately 80% of the filtered load of HCO3−. In addition, by titration of luminal pH from 7.4 down to approximately 6.7, the majority of phosphate, the major form of titratable acid, is titrated to its acid form. Lastly, ammonia synthesis occurs in the proximal tubule. Figure 11-2 shows the acid-base transport mechanisms of the proximal tubule cell. HCO3− absorption from the tubular lumen is mediated by H+ secretion across the membrane.2 This H+ secretion is active in that the electrochemical gradient favors H+ movement from lumen to cell. Two mechanisms mediate active apical H+ secretion. Approximately two thirds occurs through the apical membrane Na+-H+ antiporter NHE3.3 This protein uses the inward Na+ gradient to drive H+ secretion. The Na+-H+ exchanger has a 1 : 1 stoichiometry and is electroneutral. In parallel with the Na+-H+ antiporter, there is an apical membrane H+-ATPase that mediates approximately one third of basal proximal tubular HCO3− absorption. Both these H+ transporters generate base in the cell, which must exit across the basolateral membrane to effect transepithelial transport. This primarily occurs through a basolateral Na+-HCO3−-CO32− cotransporter. Because this protein transports the equivalent of two net negative charges, the negative cell voltage generated by the basolateral Na+,K+-ATPase provides a strong favorable driving force for base efflux. The Na+ that is carried on this transporter is moved out of the cell energy free in that ATP is not required. The Na+-3HCO3− cotransporter NBC1, encoded by the gene SLC4A4, mediates the majority of proximal tubule basolateral base exit.4 Carbonic anhydrase II is present in the proximal tubular cell cytoplasm, and carbonic anhydrase IV is on the apical and basolateral membranes. Carbonic anhydrase (carbonate dehydratase) has a number of functions in the proximal tubule. Apical membrane carbonic anhydrase allows secreted H+ ions to react with luminal HCO3−, forming H2CO3, which rapidly dissociates to CO2 + H2O. This CO2
Normal Acid-Base Balance
Definition
Net Acid Production
Buffer Systems in Regulation of pH
Respiratory System in Regulation of pH
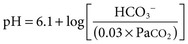
Renal Regulation of pH
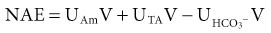
Renal Transport Mechanisms of Hydrogen and Bicarbonate Ions
Glomerulus
Proximal Tubule
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Normal Acid-Base Balance
Chapter 11