Etienne Macedo, Josée Bouchard, Ravindra L. Mehta
Prevention and Nondialytic Management of Acute Kidney Injury
Acute kidney injury (AKI) acquired in the hospital is often a result of a combination of insults. The most commonly associated causes are failure of renal autoregulation, direct nephrotoxicity, ischemia-reperfusion, and inflammatory states. AKI severity predicts adverse outcomes, such as requirement for renal replacement therapy (RRT), length of hospital stay, and mortality. In addition, the widespread use of the RIFLE and Acute Kidney Injury Network (AKIN) classification systems (see Chapter 71) has shown that even small changes in creatinine levels are associated with short- and long-term increased mortality.1–6 Furthermore, the distant effects of AKI contribute to dysfunction of other organs, such as the heart, lung, brain, and liver. Consequently, primary prevention and early diagnosis of AKI are of central clinical importance. Once a decline in glomerular filtration rate (GFR) has been detected, secondary prevention to attenuate the effects of injury and treatment of the consequences of injury are necessary.
Risk Assessment
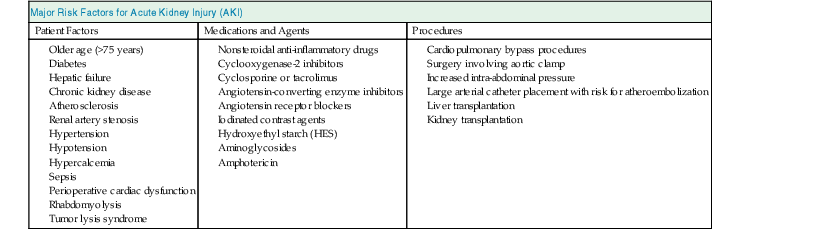
Considering the conceptual model of AKI illustrated in Figure 73-1, the first step in preventing AKI is an adequate risk assessment. The initial care of patients at risk should be focused on identification and, if possible, reversal of the risk factors. Determination of baseline kidney function is fundamental for assessing the risk of AKI in hospitalized patients. However, baseline creatinine is not available for most patients, and the first creatinine measured in the hospital is likely to be affected by the disease process that occurred before hospital admission. This problem reflects the need for more sensitive and specific biomarkers of cellular damage to enable early risk assessment and limit the extent of renal injury. Tables 73-1 and 73-2 summarize risk factors for AKI in different clinical settings. For a further discussion of risk factors and scoring systems, see Chapters 69 and 71.
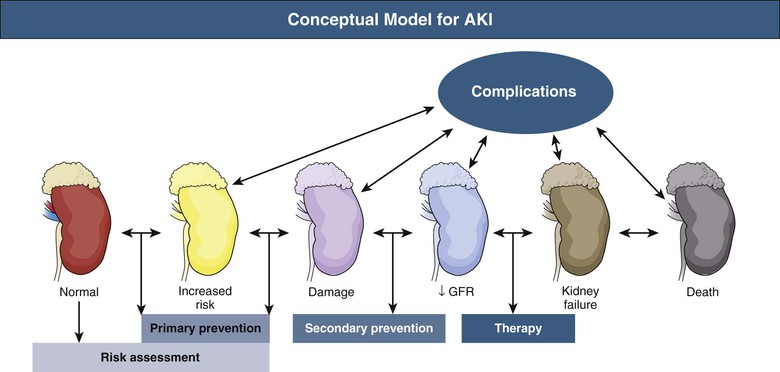
Table 73-2
Specific risk factors for the development of AKI in common clinical situations.
CABG, Coronary arterial bypass graft; COPD; chronic obstructive pulmonary disease; CVP, central venous pressure; Hct, hematocrit; IABP: intra-aortic balloon pump. *A-a gradient, alveolar-arterial oxygen gradient calculated with the sea level standard formula (713 × Fio2) − (Pco2/0.8) − Pao2, where Fio2 is fractional inspired oxygen concentration, Pao2 is arterial partial oxygen pressure, and Pco2 is partial carbon dioxide pressure.
| Specific Risk Factors for the Development of Acute Kidney Injury (AKI) in Common Clinical Situations | ||
| Postoperative (General) | ||
| Miscellaneous | Hemodynamic | Gastrointestinal and Endocrine |
| Age <70 yr | Congestive heart failure | Cirrhosis/biliary surgery |
| Proteinuria | Aortic cross-clamping | Obstructive jaundice |
| Hyperglycemia | Cardiac instability | Diabetes mellitus |
| Hypertension | Major vascular surgery | |
| Massive blood transfusion | Infection/sepsis | |
| Multiorgan failure | ||
| Cardiac Surgery | ||
| Female gender | ACE inhibitor therapy | Emergency surgery |
| COPD | Heart failure | Valve surgery only |
| Proteinuria | LV ejection fraction <35% | Previous cardiac surgery |
| Preoperative SCr >2.1 mg/dl | Preoperative IABP | Other cardiac surgery |
| Anemia | Hyperglycemia | Combination of CABG + valve surgery |
| Insulin-requiring diabetes | Blood transfusion | |
| Critically Ill | Sepsis |
| High A-a gradient* | Age |
| Low serum albumin | SCr >1.3 mg/dl |
| Proteinuria | Serum bilirubin >1.5 mg/dl |
| Hyperglycemia | Elevated CVP >8 cm |
| High intraabdominal pressure | Hemodynamic instability |
| Active cancer | |
| Contrast Nephropathy | |
| Age >75 yr | Volume of contrast >100 ml |
| Heart failure | Intra-arterial injection |
| Diabetes mellitus | Systolic BP <80 mm Hg for >1 hr and need for inotropic support or IABP 24 hr after procedure |
| SCr > 1.5 mg/dl or eGFR <60 ml/min/1.73 m2 | Use of IABP |
| History of pulmonary edema | |
| Anemia/blood loss (Hct: <39% for men; <36% for women) | |
| Nephrotoxic antibiotics | |
| Aminoglycosides | Amphotericin |
| Old age | Volume depletion |
| Pre-existing renal dysfunction | Concurrent other nephropathy |
| Duration of therapy >7 days | |
| Volume depletion | |
| Divided dose regimens | |
| Liver disease | |
(Modified from reference 77.)
Primary Preventive Measures
Optimizing Volume Status and Hemodynamic Status
Regardless of the nature of an insult, hemodynamic stabilization, with optimization of the cardiac output and blood pressure (BP), is a key factor in preventing AKI. The general aims are to optimize volume status based on physiologic measurements, to maintain adequate hemodynamic status and cardiac output to ensure renal perfusion, and to avoid further insults. In the injured kidney, autoregulation of blood flow, the mechanism responsible for maintaining a constant flow during fluctuations in BP, is lost. This loss increases the susceptibility to develop AKI after episodes of hypotension. Therefore, fluid and vasoactive drug management is an important intervention for patients in the initiation or extension phase of AKI. Volume expansion can decrease the risk of AKI in the perioperative period after major vascular surgeries, renal transplantation, and procedures to correct obstructive jaundice. In these clinical scenarios, fluid volume administration is most beneficial at the initiation phase. However, once the injury is initiated and the extension phase starts, the impact of volume expansion with intravenous fluids on clinical outcomes has not been well described and needs to be balanced with the unwanted consequence of fluid accumulation and overload.
Assessment of the volume status is challenging, particularly in patients in the intensive care unit (ICU).7 In most patients the effect of fluid expansion on hemodynamic status and renal function is retrospective and frequently evaluated by trial and error. In patients in the pre-renal phase of AKI, fluid expansion can increase organ perfusion and improve renal function. In other circumstances, as in patients with severe congestive heart failure (CHF) or diastolic dysfunction, renal perfusion is inadequate despite normal volume status or volume overload. In these patients, fluid expansion can lead to worsening of cardiac function and pulmonary edema.
There are no specific guidelines for optimizing hemodynamic and fluid status for renal function preservation, but extrapolation of data from clinical settings associated with AKI can be instructive. International guidelines for sepsis management have been recently revised by the Surviving Sepsis Campaign. The recommendations include initial fluid resuscitation with crystalloids for a minimum of 30 ml/kg and adding albumin in patients who continue to require substantial amounts of crystalloid to maintain adequate mean arterial pressure (MAP).8 Fluid challenge should be continued as long as there is hemodynamic improvement, based on dynamic or static variables. Vasopressors should be initiated to maintain MAP above 65 mm Hg, and norepinephrine is the first-choice vasopressor. With regard to the kidney, there is currently no evidence that norepinephrine has a different effect on kidney function and need for RRT than vasopressin in septic patients. Inotropic agents such as dobutamine should be administered if myocardial dysfunction or ongoing signs of hypoperfusion are present.8 Late and prolonged aggressive fluid resuscitation in critically ill patients has also been associated with worse kidney outcomes and increased mortality. Therefore fluid expansion should be stopped when patients are no longer fluid responsive—not only patients with sepsis, but all patients. Data from the Fluid and Catheter Treatment Trial (FACTT) trial indicate that after initial resuscitation, a conservative approach to fluid administration is associated with faster weaning from mechanical ventilation and decreased length of ICU stay without any deterioration of kidney function or worse kidney outcomes in patients with acute lung injury.9 The Vasopressin and Septic Shock Trial (VASST) study compared the effects of vasopressin (0.01 to 0.03 U/min) to norepinephrine (5 to 15 µg/min) infusion on mortality rates in patients with septic shock and did not show any differences between groups.10 A secondary analysis from this study found that survival is optimal with a positive fluid balance of approximately 3 liters within 12 hours.11 In conclusion, a liberal fluid approach as part of early goal-directed therapy appears to be beneficial during the first hours of shock, and a conservative approach should be followed after resolution of shock. Whether these same principles apply for patients with AKI in the absence of shock is unknown. The potential risks of fluid accumulation and overload in the setting of AKI need to be considered.12
There is controversy about the optimal fluid to use for resuscitation. The recent KDIGO AKI guidelines suggest that isotonic crystalloids should be used instead of synthetic (hydroxyethyl starch [HES]) and nonsynthetic colloids (albumin) for intracellular volume expansion in patients at risk or presenting with AKI, in the absence of hemorrhagic shock.13 For albumin, the Saline Versus Albumin Fluid Evaluation (SAFE) trial of 6997 patients found that fluid resuscitation with saline or albumin resulted in similar relative risks of death in critically ill patients.14 There were also no significant differences in the proportion of patients with new single-organ and multiple-organ failure or days on RRT. Two subgroup analyses from the same study showed that use of albumin may be deleterious in patients with traumatic brain injury15 and potentially beneficial in sepsis.16 In the past, HES preparations were commonly used as nonprotein intravascular volume expanders. In addition to their efficiency in fluid management, they also have anti-inflammatory properties and reduced cost compared with albumin. However, they potentially alter coagulation and platelet function, as well as increasing the risk for AKI. The mechanism of HES-induced kidney injury may be associated with proximal renal epithelial cell uptake of HES causing an acquired lysosomal storage disease. This dose-dependent phenomenon is more pronounced in patients with impaired renal function, potentially resulting in a diffuse tissue storage and acculation of foamy-appearing macrophages.17 An experimental study with an isolated perfusion model of 10% HES 200/0.5 and 6% HES 130/0.42 compared with Ringer lactate proposed that renal interstitial proliferation, macrophage infiltration, and tubular damage are other potential pathologic mechanisms of HES-induced kidney injury.18 HES solutions are identified by three numbers, indicating the concentration of the solution, the mean molecular weight, and—the most significant one—the molar substitution (e.g., 10% HES 200/0.5 or 6% HES 130/0.4). In the past, 6% HES 130/0.4 solutions were considered safer than 10% HES 200/0.5 solutions. A recent large multicenter randomized study, including 804 patients with severe sepsis, has shown that even 6% HES 130/0.4 is detrimental to kidney function and survival compared with Ringer acetate.19 Another larger trial in 7000 ICU patients has shown that 6% HES 130/0.4 increased need for RRT but not mortality compared with 0.9% sodium chloride (saline).20 Therefore, HES should be avoided in patients at risk for and with AKI. The use of albumin may be considered when patients require substantial amounts of crystalloid to maintain adequate MAP, and its effect must be balanced against its potential risks (possibly deleterious in patients with trauma and low potential for transmitting infectious diseases).
Some animal studies have suggested that hyperchloremia resulting from 0.9% saline infusion may affect renal hemodynamics. In healthy male adults, a double-blind crossover study compared renal artery flow velocity and renal cortical tissue perfusion after a 2-liter intravenous infusion of 0.9% saline (chloride: 154 mmol/l) and a balanced buffered solution with a chloride concentration of 98 mmol/l. They showed a significant reduction in mean renal artery flow and renal cortical tissue perfusion with saline but not with the use of chloride-restrictive fluids.21 A recent retrospective study showed that chloride-restrictive fluids (lactated solution with balanced buffer–chloride concentration of 98 mmol/l or chloride-poor 20% albumin–chloride concentration of 19 mmol/l) compared with chloride-rich intravenous fluids (0.9% saline, 4% succinylated gelatin solution, or 4% albumin solution) were associated with a significant decrease in AKI incidence and RRT requirement.22 These results will need to be confirmed with other studies.
Prevention of Contrast-Induced Acute Kidney Injury
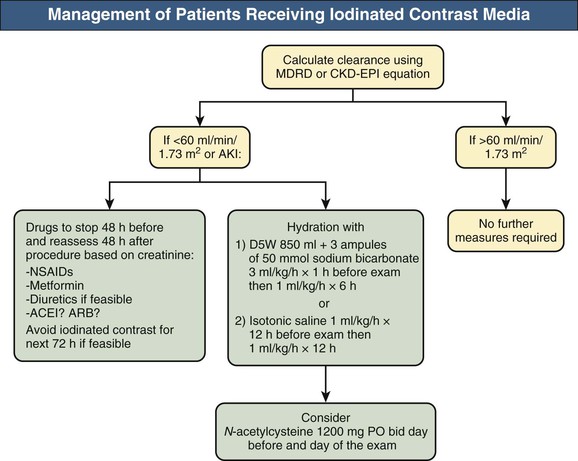
The Prevention of Contrast-Induced Acute Kidney Injury (CI-AKI) Consensus Working Panel recommends that measures to reduce the AKI risk should be implemented in patients with a baseline estimated glomerular filtration rate (eGFR) below 60 ml/min/1.73 m2. According to the KDIGO guidelines, this threshold could probably be lowered to 45 ml/min/1.73 m2. For prevention of CI-AKI, patients at risk should receive intravenous hydration (Fig. 73-2). Hydration with isotonic saline started the morning of the procedure or immediately before in cases of emergency interventions is superior to half-isotonic (0.45%) saline.23 A randomized controlled trial (RCT) compared isotonic saline with isotonic sodium bicarbonate (three 50-ml ampules of 1 mmol/ml sodium bicarbonate added to 850 ml of 5% dextrose) at 3 ml/kg/h for 1 hour before the procedure followed by 1 ml/kg/h for the 6 hours after the procedure. CI-AKI was significantly lower in the bicarbonate compared with the saline group (2% vs. 14%).24 The rationale for isotonic bicarbonate is based on animal studies showing that bicarbonate is capable of scavenging reactive oxygen species, and the increased pH in the proximal tubule and the renal medulla associated with bicarbonate administration could reduce generation of superoxide. In addition, isotonic saline contains high amounts of chloride, with a potential renal vasoconstrictor effect. Considering that most hydration studies with isotonic bicarbonate use shorter infusion protocols (7 hours) than those with isotonic saline (usually 12 to 24 hours), hydration with bicarbonate is also an attractive alternative for emergency procedures. The superiority of bicarbonate was observed in some but not all further RCTs. The KDIGO AKI guidelines recommend either isotonic sodium chloride or sodium bicarbonate solutions in patients at risk for CI-AKI unless there are contraindications to volume expansion.13 The Prevention of Serious Adverse Events Following Angiography (PRESERVE) study is an ongoing RCT (NCT01467466) with a 2 × 2 factorial design aiming to compare the effectiveness of sodium bicarbonate with isotonic sodium chloride and oral N-acetylcysteine (NAC) with placebo in 8680 high-risk patients scheduled to undergo coronary or noncoronary angiography. The study should be completed in 2016. The use of NAC is discussed later in this chapter.
Iodinated contrast medium can be categorized according to osmolality into high-osmolar contrast medium (approximately 2000 mOsm/kg), low-osmolar contrast medium (600 to 800 mOsm/kg), and iso-osmolar contrast medium (290 mOsm/kg), and clinical studies suggest that the risk of nephrotoxicity increases with increasing osmolarity of the contrast medium. The higher cost of the iso-osmolality agents bars their universal use. The KDIGO AKI guidelines recommend the use of either iso-osmolar or low-osmolar iodinated contrast medium for patients at risk of CI-AKI.
The volume of contrast material administered is also a crucial risk factor and an independent predictor of CI-AKI and should be lowered as much as possible. Based on the volume of contrast given (V) and the creatinine clearance (CrCl), a V/CrCl ratio above 3.7 has been shown to be a significant and independent predictor of CI-AKI in the general population. Administration of contrast material more than once in a short period is another risk factor, and contrast studies should be postponed at least 48 to 72 hours after the last infusion of contrast medium if possible. The drugs used for CI-AKI prevention are included in the section on pharmacologic approaches.
Prevention of Drug- and Nephrotoxin-Induced Acute Kidney Injury
Drug-induced nephrotoxicity can often be predicted because it is more common in certain patients and in specific clinical situations. Prevention involves the knowledge of mechanisms of renal injury, patient-related risk factors, and drug-related risk factors. The most important patient-related factors associated with higher risk of nephrotoxicity are age older than 60 years, preexisting chronic kidney disease (CKD), volume depletion, diabetes, heart failure, and sepsis. A fundamental step in prevention includes drug monitoring of potential nephrotoxins in at-risk patients. Preventative measures include correctly estimating the GFR before initiation of therapy, adjusting the dosage, and monitoring renal function during therapy. Alternative non-nephrotoxic drugs should be used whenever possible, and nephrotoxic drug combinations should always be avoided if feasible.
Amphotericin
Amphotericin-associated nephrotoxicity can occur in as many as one third of treated patients, and the risk of AKI increases with higher cumulative doses. Lipid formulations cause less nephrotoxicity compared with the standard formulation, and therefore amphotericin deoxycholate is preferred over conventional amphotericin; however, it is significantly more expensive. Recently, alternative antifungal agents such as itraconazole, voriconazole, and caspofungin have been more commonly used in patients at high risk for AKI and should be used rather than conventional amphotericin.
Angiotensin-Converting Enzyme Inhibitors, Angiotensin Receptor Blockers, and Nonsteroidal Anti-Inflammatory Drugs
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) cause vasodilation of the efferent glomerular arteriole, further reducing intraglomerular pressure already compromised by the BP-lowering effect of these agents. In patients with renal dysfunction, they can contribute to a reduction in GFR. In patients with an increase in serum creatinine higher than 30% after the initiation of ACE inhibitor and ARB treatment, bilateral renal artery stenosis, stenosis of the renal artery in a solitary kidney, diffuse intrarenal small-vessel disease, or generalized volume depletion should be suspected, and these drugs should be discontinued.
Nonsteroidal anti-inflammatory drugs (NSAIDs) should be used with caution in patients with atherosclerotic cardiovascular diseases (CVDs). NSAIDs should be avoided in CKD and intravascular volume depletion because they inhibit cyclooxygenase, which blocks prostaglandin-induced vasodilation of the afferent arteriole, potentially reducing GFR and renal blood flow. In critically ill patients, renal hypoperfusion caused by decreased effective circulating volume is relatively common, and inhibition of prostaglandin-induced vasodilation may further compromise renal blood flow and exacerbate ischemic injury.
Aminoglycosides
Acute kidney injury caused by aminoglycoside nephrotoxicity usually occurs 5 to 10 days after initiation of the treatment; this type of AKI is typically nonoliguric and associated with decreased urine concentrating ability and urinary magnesium wasting. Because of nephrotoxicity, ototoxicity, and vestibular toxicity, the AKI KDIGO guidelines have recommended avoiding the use of aminoglycosides in patients with AKI and those at risk unless no other alternative is available. With multiple daily administration schedules, elevated aminoglycoside peak levels appear to correlate with nephrotoxicity. Because aminoglycoside uptake by proximal tubular cells is a saturable process, once-daily administration can decrease tubular cell toxicity by reducing drug taken up by proximal tubular cells. In the general population, extended intervals between doses maintains the target dose while decreasing the risk of nephrotoxicity compared with multiple daily doses; therefore, in patients with normal kidney function who are not at risk for AKI, aminoglycosides should be administered daily if needed.
Tumor Lysis Syndrome
Tumor lysis syndrome (TLS) is caused by uric acid and calcium-phosphate precipitation in the tubules. Correct identification of those at high risk is the first step toward preventing AKI in this setting. In patients with high-grade hematologic malignant neoplasms, risk factors for TLS are lactate dehydrogenase levels above 1500 IU, large tumor burden, extensive bone marrow involvement, CKD, and high tumor sensitivity to chemotherapeutic agents. In patients with low or intermediate risk of TLS, a xanthine oxidase inhibitor such as allopurinol can be used as a hypouricemic agent and should be started 2 days before chemotherapy. Aggressive hydration with isotonic saline is initiated 2 days before the chemotherapy to maintain a high urinary output, allowing the elimination of uric acid and phosphate. If urinary output decreases despite adequate fluid intake, a loop diuretic should be added, but RRT will be required if oliguria persists.25 The use of urine alkalinization to promote elimination of urates is not recommended because it can induce calcium phosphate deposition and therefore aggravate TLS. In addition to the hydration, recombinant urate oxidase can reduce uric acid levels and the risk of uric acid deposition nephropathy.26 Recombinant urate oxidase should be initiated in high-risk patients or for established TLS when hyperuricemia is severe.
Secondary Prevention
After the renal insult has occurred, secondary preventive measures should be directed to avoid further injury, to facilitate repair and recovery, and to prevent AKI complications. The timeliness of interventions is crucial to their effectiveness for secondary prevention. Various approaches have been applied but are best appreciated in the context of specific clinical scenarios.
Traumatic and Nontraumatic Rhabdomyolysis
In the prevention of myoglobin-induced nephropathy after crush syndrome, intravenous hydration should be initiated with isotonic saline before the crushed limb is relieved to prevent precipitation of the pigment in the tubular lumen. A solution with 2.7% sodium bicarbonate (50 mmol/l) should be given every second or third liter to maintain urinary pH above 6.5 and to prevent intratubular deposition of myoglobin and uric acid. The urine output should be maintained around 300 ml/h, which may require an infusion of up to 12 liters of fluid per day. The volume administered is generally much greater than the urinary output; the accumulation of fluid in the damaged muscles may exceed 4 liters. This protocol should be continued until clinical or biochemical evidence of myoglobinuria disappears, usually by day 3. It has also been suggested that mannitol is beneficial because of its diuretic, antioxidant, and vasodilatory properties. Mannitol could prevent renal tubular cast deposition, expand extracellular volume, and reduce intracompartmental pressure, muscle edema, and pain.15 However, mannitol may exacerbate CHF and nephrotoxicity, requires close monitoring, and is contraindicated in oliguria, hypervolemia, hypertension, and heart failure. Mannitol administration is considered, if urinary flow is sustained above 20 ml/h, given at a rate of 5 g/h added to each liter of infusate and not exceeding 1 to 2 g/kg/day.27 Muscle damage induces stretch-activated ion channels, allowing for influx of calcium into cells after reperfusion. The resultant hypocalcemia is usually asymptomatic but can lead to cardiac dysrhythmias. Hence, care must be taken to avoid an NaHCO3 (sodium bicarbonate)–induced decrease in ionized calcium (caused by metabolic alkalosis), which can trigger tetany, seizures, and cardiotoxicity and worsen existing muscle damage. During the AKI recovery phase, hypercalcemia is frequent, mainly in patients who received calcium infusion, as a result of the mobilization of previously precipitated calcium in muscles. Thus, hypocalcemia should be treated only if symptomatic. The importance of early fluid administration and the most important aspects of the treatment of crush victims have been recently summarized.28
In nontraumatic rhabdomyolysis, prevention of AKI involves vigorous fluid expansion to maintain renal perfusion pressure and to dilute myoglobin and other toxins. A urine output of 200 to 300 ml/h is desirable until myoglobinuria disappears. Urine alkalinization may help prevent tubular pigment cast formation; however, there is no clinical evidence that mannitol and bicarbonate are more effective than saline solution alone. Furthermore, there are potential risks to bicarbonate therapy, including precipitation of calcium phosphate and hypocalcemia.
In treating patients with rhabdomyolysis, it is important to consider when to stop aggressive fluid resuscitation. Although fluid expansion is the main therapeutic intervention to reduce the hemoglobin precipitation in the tubular lumen, the risk of fluid accumulation and compartmental expansion should always be part of the clinical judgment. Frequent assessment of renal functional parameters (e.g., every 6 to 12 hours) associated with uric acid and creatine kinase levels help the clinician decide how intense the volume expansion should be.