Time post-transplant
Type of transplant: living donor or cadaveric
Donor history if pertinent
Infection including history of HIV, hepatitis C, etc.
Peritransplantation surgical complications
Indication for biopsy
Increase in serum creatinine (rapid, slow)
Type of immunosuppression
Changes in immunosuppression
Use of nephrotoxic drugs
Urine culture results
Proteinuria
Dehydration
Renal artery stenosis
Abnormal imaging studies (i.e., hydronephrosis)
Prevention, diagnosis, and treatment of antibody-mediated rejection (AMR) require monitoring for the presence of circulating donor-specific antibodies (DSAs) at regular intervals after transplantation, as well as at the time of biopsy and whenever rejection is suspected [2–4]. As discussed in detail below, accurate classification of rejection according to the Banff schema requires correlation between the biopsy findings and DSA studies [2].
Biopsy Adequacy
According to the Banff schema a specimen must contain ten or more glomeruli and at least two arteries to be considered adequate [5].
Due to the fact that many pathological processes involve the allograft in a patchy, random manner, the sensitivity of the biopsy depends on the amount of tissue available for evaluation. To diagnose or rule out acute allograft rejection with some degree of certainty, it is necessary to evaluate a sample of renal cortex containing sufficient number of glomeruli and arteries. Evaluation of two tissue needle cores is desirable to achieve a sensitivity of 99 % for the diagnosis of acute rejection. This is in contrast to a sensitivity of 90 % for a single core [6, 7]. Biopsies containing only medulla are in general considered inadequate because in those samples, acute allograft rejection will be missed or underestimated in half of the cases [8]. On the other hand, examination of renal medulla may be sufficient to diagnose other pathological entities (i.e., acute pyelonephritis, polyomavirus nephropathy) (PVN) [9].
The amount of tissue required also depends on the biopsy indication. There are clinical situations in which enough tissue material must be obtained to allow for the performance of ancillary studies such as immunofluorescence and electron microscopy (EM). This is particularly important if the biopsy is performed for a possible glomerular disease.
Routine Histological Studies
Light microscopic evaluation of renal biopsies includes the performance of hematoxylin and eosin (H&E)-stained sections (Fig. 31.1), as well as a set of routinely used special stains (periodic acid stain [PAS], periodic acid methenamine silver stain (Jones stain) [PAMS], Masson’s trichrome stain [MT], and C4d complement fragment [C4d] stain) [4, 5]. Table 31.2 summarizes the main morphological features and diagnostic associations identified with each of these stains (Fig. 31.2). The C4d stain is required for the identification of an important subset of AMRs [4, 10, 11]. C4d is a complement split product that remains covalently bound to target structures after complement activation has occurred, allowing for its recognition in tissue sections. This is in contrast to most products of complement activation that are immediately degraded and cannot be detected on standard tissue processing. Deposition of C4d in peritubular capillaries (PTC) identifies ongoing humoral rejection that may otherwise escape identification. The C4d stain can be performed in fresh-frozen as well as formalin-fixed tissue with comparable results [4].
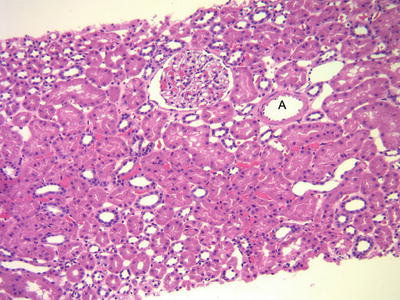
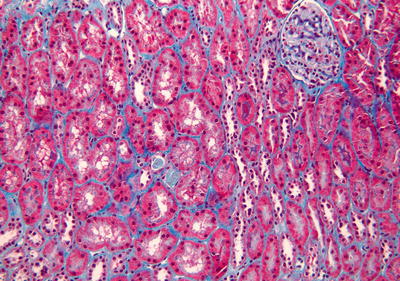

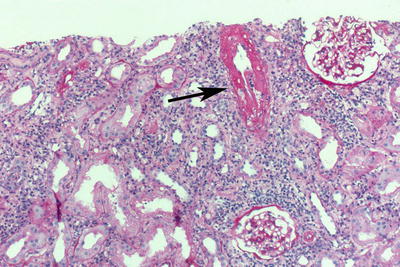
Fig. 31.1
H&E-stained biopsy from a graft with excellent function (protocol biopsy at 12 months). There is no inflammation. The tubules are “back to back” with no significant intervening connective tissue. A normal arteriole. Note the thin wall and the widely open lumen
Table 31.2
Routinely* used special stains: main diagnostic value
PAS* |
Tubules: Tubulitis, destruction of tubular basement membranes, thickening of basement membranes (atrophy) |
Glomeruli: Glomerulitis (mononuclear cells in capillaries), reduplication of basement membranes (transplant glomerulopathy), increase in mesangial matrix, hyalinosis |
Vessels: Hyalinosis |
Other: Identification of fungal organisms |
Silver stain (PAMS, Jones stain)* |
Tubules: Tubulitis, destruction of tubular basement membranes, thickening of basement membranes (atrophy) |
Glomeruli: Glomerulitis (mononuclear cells in capillaries), reduplication of basement membranes (transplant glomerulopathy), increase in mesangial matrix |
Trichrome stain* |
Collagen deposition: Interstitial fibrosis, arterial sclerosis, glomerular sclerosis, fibrin thrombi, fibrinoid necrosis |
C4d stain* |
Staining of peritubular capillaries in AMR (also see ref. [10]) |
Immunohistochemical stains for viral infections |
Cytomegalovirus |
SV40 (cross reacts with BK virus and JC virus) |
EBV latent membrane protein (LMP-1) |
EBER—Epstein–Barr-encoded RNA (in situ Hybridization) |
Adenovirus |

Fig. 31.2
Masson’s trichrome-stained biopsy from a well functioning graft. Stain for collagen highlights minimal connective tissue between tubules. There is no evidence of tubular atrophy or interstitial fibrosis
Main Ancillary Studies
Infectious Processes
In addition to standard serological studies for infectious processes (i.e., CMV, hepatitis C, HIV), immunohistochemical stains and in situ hybridization are used for the identification of a variety of infectious organisms in tissue: SV40 (simian virus 40 stain for BK and JC polyomaviruses), CMV (for cytomegalovirus), EBV (for Epstein–Barr virus/post-transplant lymphoproliferative disorder [PTLD]), immunostains for adenovirus, etc. Stains for acid-fast bacilli and fungi are performed in biopsies with granulomatous inflammation or if these infections are suspected [1]. Molecular studies, particularly PCR, are also useful to demonstrate the presence of infectious organisms in tissue samples although these studies are not routinely used for diagnostic purposes.
Routine determination of polyomavirus BK viral loads in plasma and urine is essential for prevention and monitoring of polyomavirus allograft nephropathy (PVN). Evaluation of routine urine cytology with identification of the PV-infected “decoy cells” is an inexpensive and simple tool to determine if a patient has significant PV replication in the urinary tract. Persistent PV BK urinary shedding is associated with PVN and has markedly increased risk of graft loss [14, 15].
Electron microscopy studies and immunofluorescence stains (IF; for immunoglobulins and complement components) are required for the evaluation of post-transplant glomerular processes in the same manner as it is done in native kidney biopsies. Furthermore, ultrastructural studies of glomeruli and PTC are essential for the diagnosis of early AMR in order to demonstrate the characteristic basement membrane abnormalities.
It is important to remember that the immunofluorescence technique can be applied only to tissues that are fresh-frozen (i.e., not fixed in formaldehyde).
Pretransplant Biopsy
Deceased Donor Evaluation (Harvest Biopsies)
Routine use of kidneys from less than ideal donors requires pretransplant histological evaluation for determination of organ quality. Pretransplant assessment is indicated for kidneys from older donors, or from those with history of hypertension, diabetes, or renal dysfunction (extended criteria donors) [16].
Hypertensive Disease
The most common donor-related histological abnormalities are secondary to long-standing arterial hypertension [17]. The large and small arteries show sclerosis and sclerosis with hyalinosis, respectively, leading to various degrees of luminal narrowing (Fig. 31.3). The interstitium shows increased fibrosis and there is corresponding atrophy of the tubular components. Subcapsular ischemic scars as well as a significant number of scattered obsolescent glomeruli are also indicative of chronic hypertension (nephrosclerosis). The Maryland aggregate pathology index (MAPI) is a histological scoring system based on the presence or absence of five morphological features associated with hypertensive/chronic ischemic renal parenchymal injury (hyalinosis of glomerular-size arterioles, >15 % global glomerulosclerosis, significant arterial sclerosis, periglomerular fibrosis, and cortical scar) which are assigned scores according to their relative significance. The MAPI has been shown to be predictive of post-transplant graft survival or failure [16].
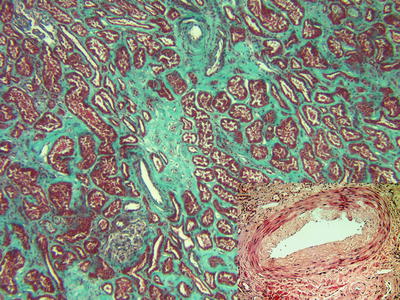

Fig. 31.3
Masson’s trichrome-stained biopsy from a donor with chronic hypertensive disease (extended criteria donor). The tubules are separated by an abnormal amount of connective tissue which is highlighted by the collagen stain. An arteriole with a thick wall and narrowed rounded lumen is noted in the top center. The H&E-stained insert demonstrates an interlobular artery with fibrointimal sclerosis (arteriosclerosis)
Donor Diabetes
Approximately 4 % of all kidney transplants in the United States are derived from diabetic donors [17, 18]. Identification of fully developed nodular diabetic glomerulosclerosis in a donor kidney biopsy is a contraindication for transplantation; however, milder diabetic glomerular involvement is compatible with acceptable outcomes [18, 19].
Renal Cell Carcinoma
With the increased utilization of organs from older donors there has been an increase in the identification of renal cell carcinomas both in deceased and living donors (≈1 %). Organs with small, completely resected, low grade tumors are now routinely utilized for transplantation with excellent outcomes [20]. Frozen section evaluation is required for rapid histological characterization diagnosis of any donor mass. In this setting, the two most common lesions are small renal cell carcinomas and angiomyolipomas. The latter are benign connective tissue tumors that do not pose any risk to the recipient irrespective of their size [21].
Other Donor-Related Issues
A variety of other processes can be identified in pretransplant donor biopsies, one of the most common being mesangial IgA deposition and membranous nephropathy, both of which appear not to have a negative post-transplantation impact. Similarly, other forms of mild glomerulonephritis can resolve after transplantation [22]. In addition, we have seen successful outcomes after transplantation of kidneys with mild sarcoidosis and eclampsia-related glomerular injury. The decision about transplanting organs with known pathological processes is made after careful consideration, on a case-by-case basis.
Living Donor Evaluation
Living donors may present with potentially significant renal disease requiring careful evaluation before donation. The risk factors for renal disease associated with living-donation have been characterized [23]. Kidney biopsies are more often performed for hematuria in otherwise healthy living donors. In this context the identification of thin basement membrane disease is common; in general terms this process is not considered per se a contraindication for donation.
Post-implantation Biopsies: Time 0 Biopsy
Similar to donor harvest biopsies, baseline graft biopsies performed before or after reperfusion provide essential information about the status of the organ at the time of transplantation, in particular regarding the presence of underlying chronic vascular disease (donor hypertensive disease) [22]. Microvascular and/or glomerular thrombosis in implantation biopsies correlates with ischemic injury and was associated with higher risk of rejection in one study [24].
Biopsies for Graft Dysfunction in the Early Post-transplant Period
Delayed Graft Function
Acute Tubular Necrosis
Biopsies from patients with delayed graft function (DGF) in the early post-transplant period usually show acute tubular necrosis (ATN). This injury is presumed to be ischemic in nature as a result of the organ procurement procedures and reperfusion injury. Tubular injury can be subtle, consisting only of cytoplasmic vacuolization, loss of brush border, and isolated cell necrosis or apoptosis. In more pronounced cases there is frank disintegration and sloughing of the tubular cells that can accumulate as debris in the tubular lumina. When there is pronounced tubular cell loss there is flattening of the remaining tubular lining and partial denudation of the basement membranes. Tubular calcifications are common in areas of previous tubular injury (Fig. 31.4). Interstitial edema and mild interstitial inflammation, often including some eosinophils, can be present in ATN. If the inflammation is significant and there is tubulitis, a concurrent acute cellular rejection should be suspected. The presence of multifocal neutrophilic capillaritis and/or microscopic thrombi requires correlation with the C4d stain and DSA studies to rule out ATN associated with early acute AMR.
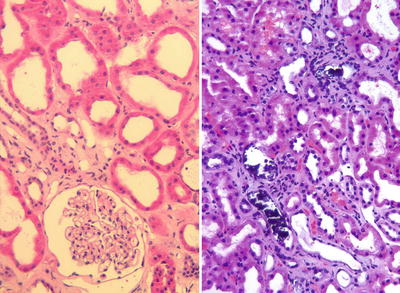

Fig. 31.4
Acute tubular necrosis. Marked dilatation of the tubular lumina and flattening (simplification) of the tubular epithelium are noted on the left image. Compare with Fig. 31.1 where the normal tubular cells are tall and fill most of the luminal space. The image on the right shows features of tubular injury and scattered tubular microcalcifications, which tend to develop in areas of tubular necrosis
Coagulation Necrosis: Infarction
Severe ischemic injury often secondary to hemodynamic issues or surgical complications may result in focal of diffuse parenchymal necrosis. Localized infarcts are often unsuspected clinically and may become evident only in a needle biopsy for evaluation of DGF. Identification of infarction in core biopsies is usually associated with significant ischemic injury and always leads to subsequent fibrous scarring of the infarcted areas (Fig. 31.5). Therefore, depending on the extent of parenchyma involved, infarcts may be associated with poor graft function or primary non-function.
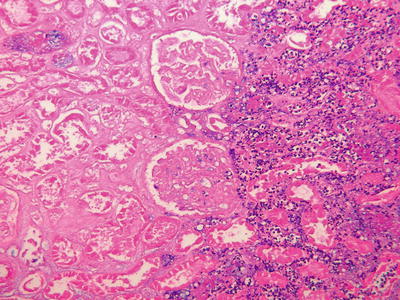

Fig. 31.5
Cortical infarct. Ischemic necrosis of the renal cortex is appreciated on the left side of the image (loss of nuclear profiles). On the right a band of neutrophilic infiltrates demarcates the edge of the infarct
Ischemic Endothelial Injury
In patients with DGF, glomerular endothelial cell swelling and microthrombi that may mimic early acute AMR may result from ischemic injury to endothelial cells [25, 26] (Fig. 31.6). These changes are usually reversible unless the ischemic process has also led to multifocal areas of infarction. In patients with microvascular changes suspected to represent ischemic endothelial injury, examination of a C4d stain and serological studies to document the absence of DSA are necessary to confirm the purely ischemic nature of the injury.
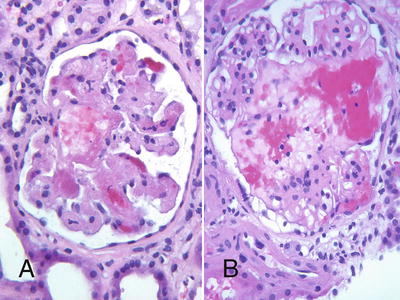

Fig. 31.6
Thrombotic microangiopathy (TMA) leading to glomerular necrosis in a pre-sensitized patient with early acute AMR (a), and similar changes due to ischemic endothelial injury in a patient with prolonged cold ischemia time (b)
Preexisting Donor Hypertensive Vascular Disease
Suboptimal Graft Function
Suboptimal or slow graft function is a common indication for early KTxBx in patients that do not require dialysis but that continue to have poorer than expected renal function. The most common pathological findings in these patients are tubular injury (i.e., tubular cell vacuolization, ATN-like changes, etc.), and donor-related issues, particularly related to chronic hypertensive donor disease (e.g., patches of cortical scarring, vascular sclerosis, and arteriolar hyalinosis) or less commonly related to preexisting conditions such as donor diabetes. In patients with persistent tubular injury, ongoing ischemia (i.e., stenosis of the renal artery anastomosis), urinary obstruction, and drug toxicity should be ruled out clinically.
Acute calcineurin inhibitor (CNI) toxicity can be the cause of suboptimal graft function in the early post-transplant period. Isometric tubular vacuolization (finely vacuolated cytoplasmic droplets), a feature typically associated with acute CNI toxicity, is not specific and can be observed in most other forms of tubular injury including ischemic injury and in osmotic nephrosis (Fig. 31.7). A more specific lesion associated with high levels of CNI consists of ballooning, apoptosis or necrosis, and drop-out of arteriolar myocytes. These uncommon changes are dose related and are typically reversible with dose adjustments [27] (Fig. 31.8).
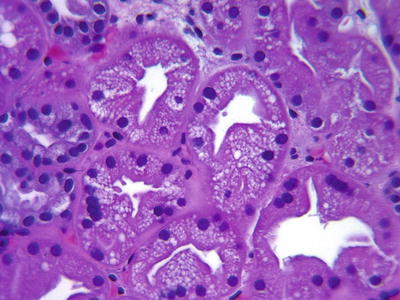
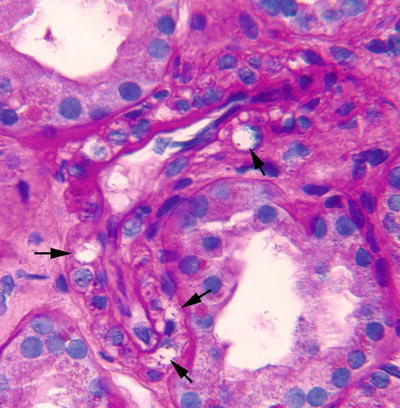

Fig. 31.7
Isometric tubular vacuolization. The tubular cells have a “foamy,” clear cytoplasm with accumulation of multiple vacuoles. Tubular injury secondary to multiple etiologies (i.e., calcineurin inhibitor [CNI] toxicity, ischemia, osmotic nephrosis) can manifest with this change

Fig. 31.8
Myocyte injury due to CNI toxicity. High power view of a pre-glomerular arteriole shows marked vacuolization of some myocytes (arrows). Some of the clear spaces represent myocyte cell necrosis (drop-out); note scattered granular structures consistent with nuclear fragments/apoptotic bodies
Acute Allograft Rejection
Early Acute AMR
The most characteristic lesions of early acute AMR result from lytic endothelial cell graft injury due to damage by alloantibodies (i.e., anti-HLA DSA).
Specifically, circulating DSA in conjunction with complement activation leads to endothelial cell swelling and necrosis with superimposed formation of microthrombi. Accumulation of inflammatory cells (neutrophils and macrophages) in glomerular and PTC appearing as glomerulitis and capillaritis is also a common feature of acute AMR [4].
Early acute AMR develops in a proportion of pre-sensitized patients despite conditioning protocols and may also occur in the early post-transplantation period in patients with no apparent risk for AMR [30].
In contrast to milder forms of early acute AMR which are characterized by attenuated endothelial injury that may even resemble bland ATN, in hyperacute rejection, the most severe form of AMR, there are abundant preformed circulating antibodies leading to diffuse arterial and venous necrosis with secondary thrombosis and parenchymal infarction [4]. A diagnosis of pure acute AMR requires exclusion of features of T-cell-mediated rejection (CMR).
The current Banff classification of acute AMR is based on the combination of (1) C4d+, (2) the presence of circulating antidonor antibodies, and (3) morphologic evidence of acute tissue injury (see Table 31.3 [4, 31]). Acute AMR is categorized in types or grades I, II, or III (I. ATN-like minimal inflammation, II. capillary and or glomerular inflammation and/or thromboses, and III. arterial necrosis). In essence, the Banff Category I (C4d+ and ATN-like changes only) represents a milder form of Category II (characterized additionally by microvascular inflammation and microthrombi). Category III is defined by arterial fibrinoid necrosis resembling lesions seen in hyperacute rejection and therefore represents a more severe form of AMR in comparison to Categories I and II.
Table 31.3
Banff 97 diagnostic categories for renal allograft biopsies—Banff ‘09 update
1. Normal |
2. Antibody-mediated changes (may coincide with categories 3, 4 and 5 and 6) |
Due to documentation of circulating antidonor antibody, C4da, and allograft pathology |
C4d deposition without morphologic evidence of active rejection |
C4d+, the presence of circulating antidonor antibodies, no signs of acute or chronic CMR or AMR (i.e., g0, cg0, ptc0, no ptc lamination (<5 layers by electron microscopy), no ATN-like minimal inflammation). Cases with simultaneous borderline changes are considered as indeterminate |
Acute antibody-mediated rejection b |
C4d+, the presence of circulating antidonor antibodies, morphologic evidence of acute tissue injury, such as (type/grade) |
I. ATN-like minimal inflammation |
II. Capillary and/or glomerular inflammation (ptc/g >0) and/or thromboses |
III. Arterial—v3 |
Chronic active antibody-mediated rejection b |
C4d+, the presence of circulating antidonor antibodies, morphologic evidence of chronic tissue injury, such as glomerular double contours and/or peritubular capillary basement membrane multilayering and/or interstitial fibrosis/tubular atrophy and/or fibrous intimal thickening in arteries |
3. Borderline changes: “Suspicious” for acute T-cell-mediated rejection (may coincide with categories 2 and 5, and 6) |
This category is used when no intimal arteritis is present, but there are foci of tubulitis (t1, t2, or t3) with minor interstitial infiltration (i0 or i1) or interstitial infiltration (i2, i3) with mild (t1) tubulitis |
4. T-cell-mediated rejection (CMR, may coincide with categories 2 and 5 and 6) |
Acute T-cell-mediated rejection (type/grade): |
IA. Cases with significant interstitial infiltration (>25 % of parenchyma affected, i2 or i3) and foci of moderate tubulitis (t2) |
IB. Cases with significant interstitial infiltration (>25 % of parenchyma affected, i2 or i3) and foci of severe tubulitis (t3) |
IIA. Cases with mild to moderate intimal arteritis (v1) |
IIB. Cases with severe intimal arteritis comprising >25 % of the luminal area (v2) |
III. Cases with “transmural” arteritis and/or arterial fibrinoid change and necrosis of medial smooth muscle cells with accompanying lymphocytic inflammation (v3) |
Chronic active T-cell-mediated rejection |
“Chronic allograft arteriopathy” (arterial intimal fibrosis with mononuclear cell infiltration in fibrosis, formation of neo-intima) |
5. Interstitial fibrosis and tubular atrophy, no evidence of any specific etiology (may include nonspecific vascular and glomerular sclerosis, but severity graded by tubulointerstitial features) |
Grade |
I. Mild interstitial fibrosis and tubular atrophy (<25 % of cortical area) |
II. Moderate interstitial fibrosis and tubular atrophy (26–50 % of cortical area) |
III. Severe interstitial fibrosis and tubular atrophy/loss (>50 % of cortical area) |
6. Other: Changes not considered to be due to rejection—acute and/or chronic (for diagnoses see table 14 in [49]; may include isolated g, cg, or cv lesions and coincide with categories 2, 3, 4, and 5) |
Differential diagnosis of acute AMR: The most important differential diagnosis of early acute AMR is ischemic injury leading to microvascular thrombosis [25, 26] (Fig. 31.6). History of prolonged cold ischemia time and/or surgical complications may support the impression of ischemic graft injury; however, evaluation of C4d staining and DSA studies are essential to rule out a concurrent component of acute AMR. In our experience some degree of C4d staining may be present in early biopsies with ischemic injury, but this typically resolves in subsequent biopsies. Renal function recovers in most patients with ischemic endothelial injury, unless there is very extensive microvascular thrombosis or infarcts which would eventually lead to diffuse glomerular sclerosis and formation of scars. TMA of other etiologies—including CNI toxicity—may also require differentiation from acute AMR (see section “Thrombotic Microangiopathy”).
Acute CMR
Acute CMR is characterized by interstitial T-cell and macrophage infiltrates, tubular inflammation (tubulitis), and inflammation of the arterial endothelium (intimal arteritis). A diagnosis of pure acute CMR requires exclusion of AMR (evaluation of C4d stain and negative DSA studies).
The current Banff classification of acute CMR uses the following categories: Borderline—suspicious, Type I (A and B), Type II (A and B), and Type III (see Table 31.3).
Borderline changes: “Suspicious” for acute CMR is used when no intimal arteritis is present, but there are foci of tubulitis with minor interstitial infiltration or interstitial infiltration with only mild tubulitis.
On the other hand, Types I–III are considered diagnostic of acute CMR. In essence, Type I rejection is defined by tubulitis (Fig. 31.9), Type II is defined by arterial inflammation without necrosis (intimal arteritis, endarteritis) (Fig. 31.10), and Type III is characterized by arterial necrosis and accompanying lymphocytic inflammation (Fig. 31.11). Whereas Type II CMR (intimal arteritis) is the most diagnostic form, Type I requires differentiation from other causes of graft inflammation (i.e., PVN, infections, nonspecific inflammation) and Type III requires differentiation from AMR due to overlap of necrotizing vascular lesions in the severe forms of CMR and AMR.
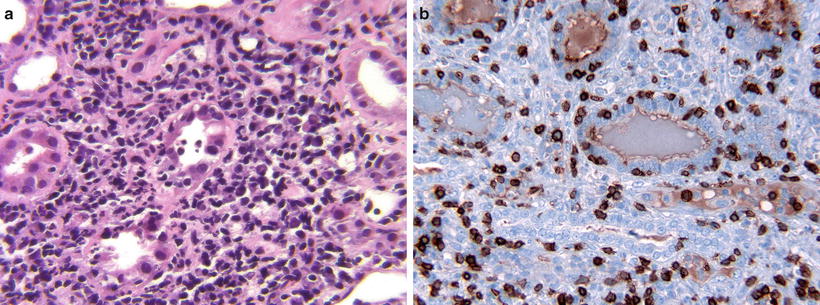
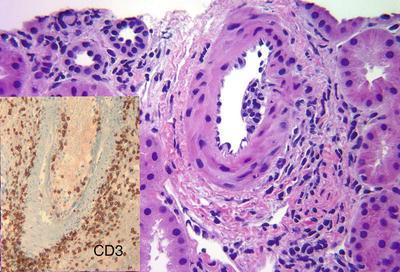

Fig. 31.9
(a) Acute CMR (Type I). The interstitium contains dense, active inflammatory infiltrates. The tubule in the center is partially destroyed by severe tubulitis. (b) Acute CMR with severe tubulitis (Type 1B). CD3 demonstrates numerous T-lymphocytes that have permeated the tubular basement membranes

Fig. 31.10
Acute CMR (Type II). Interlobular artery with intimal arteritis characterized by subintimal accumulation of lymphocytes and associated endothelial cell damage. Inset: CD3 stain highlights T-lymphocytes in the intimal arteritis. Interstitial perivascular inflammation is also prominent