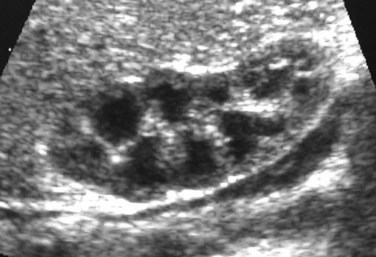
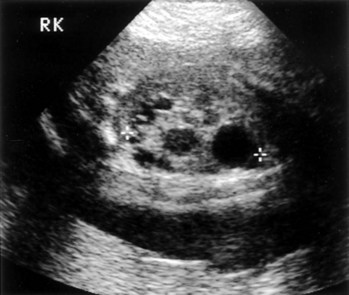
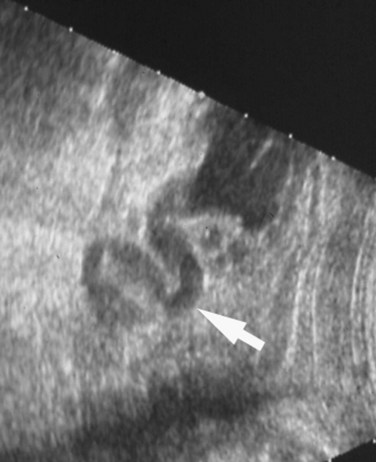
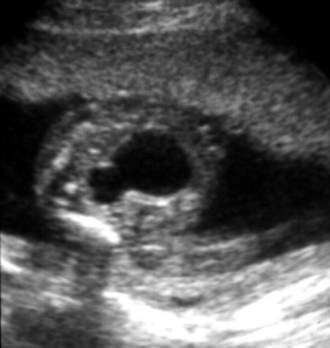
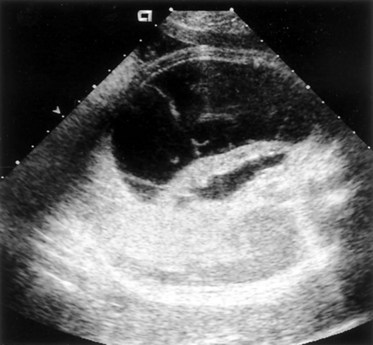
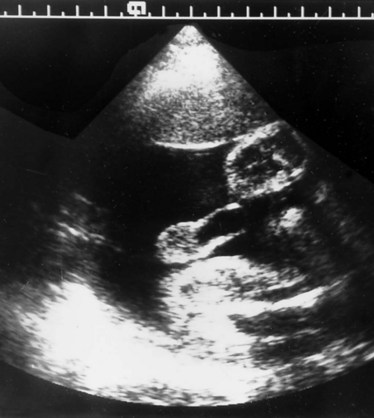
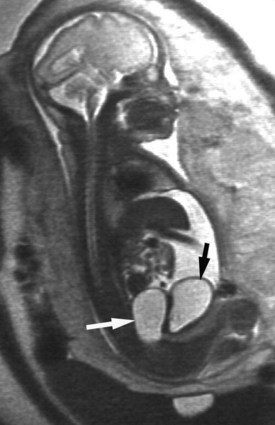
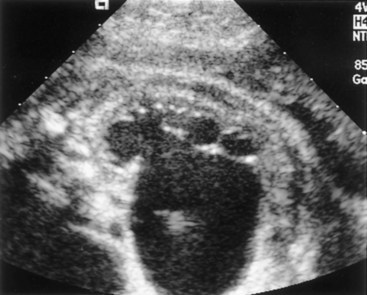
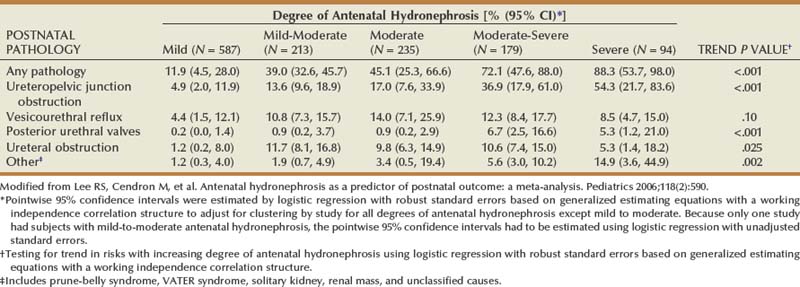
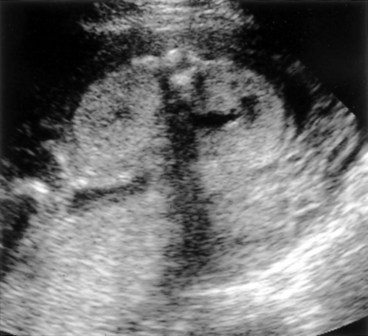
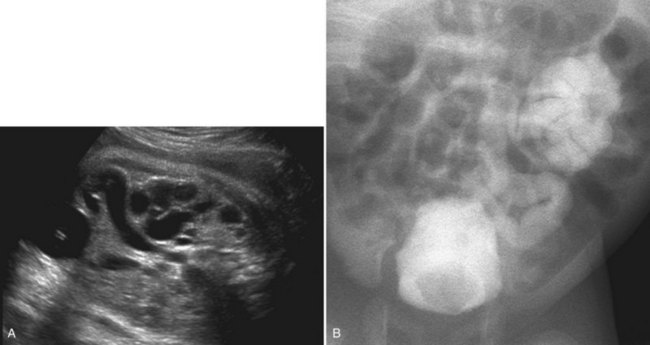
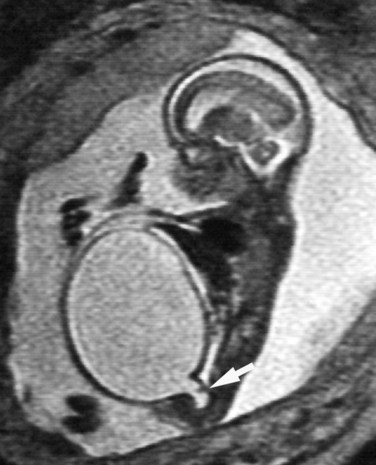
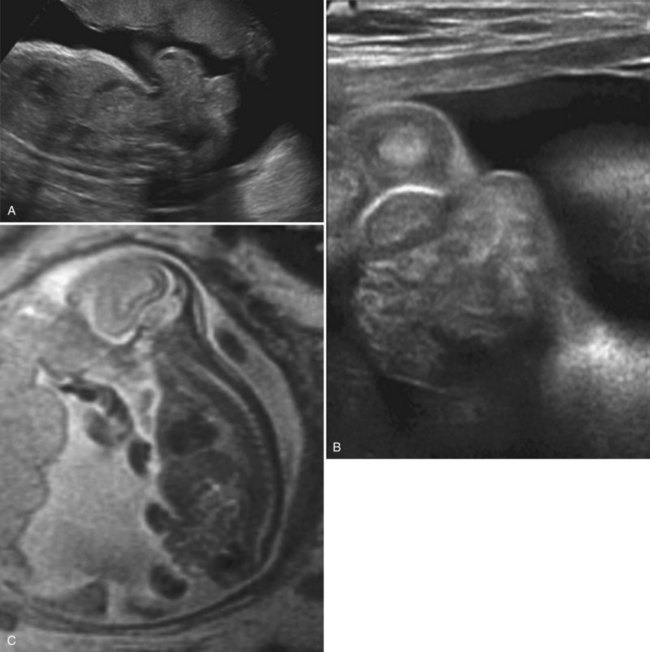
Richard S. Lee, MD, Joseph G. Borer, MD The increased use of maternal-fetal ultrasound has led to the development of the field of perinatal urology. Antenatal hydronephrosis (ANH) is identified in 1% to 3% of all pregnancies and is one of the most common birth defects detected (Livera et al, 1989; Blyth et al, 1993; Gunn et al, 1995; Sairam et al, 2001). In addition to hydronephrosis, renal cystic disease, renal agenesis, stones, and tumors have been diagnosed prenatally as well. For the urologist, these prenatal findings have created numerous challenging clinical and scientific dilemmas. Fetal magnetic resonance imaging (MRI) is a valuable adjunct in those instances in which further delineation of anatomic detail is believed to be necessary to optimize diagnosis and/or management strategy (Estroff, 2009). As with ultrasonography there is no radiation exposure. Utilization of complementary computed tomography is controversial, because the additive information may not outweigh the added risk of fetal and maternal radiation exposure. Previously, a large prospective study of 11,986 Swedish women done between 1978 and 1983 identified renal anomalies in 0.28% of fetuses, of which over two thirds of the anomalies were hydronephrosis (0.18%) (Helin and Persson, 1986). Similarly, a British prospective screening study of 6292 pregnant women at 28 weeks’ gestation demonstrated hydronephrosis in 1.40% of cases, with postnatal confirmation in 0.65% (Livera et al, 1989). These authors defined ANH as an anteroposterior diameter (APD) of the renal pelvis greater than 5 mm but noted the lack of consensus on the definition of ANH (Scott and Renwick, 1993, 1999; Scott et al, 1995). With the rapid improvement of ultrasound technology the incidence of detection of renal anomalies may be changing. In a more recent prospective cohort study (1999-2003), a 0.76% incidence of urinary tract abnormalities was detected that was increased as compared with an earlier cohort from the same institution (0.3%, 1989-1993) (Mallik and Watson, 2008). However, many variations in the definition and management of ANH exist in the literature and clinical practice, including method and frequency of in-utero testing, radiographic documentation, classification, or postnatal management (Benacerraf et al, 1990; Corteville et al, 1992; Fernbach et al, 1993; Adra et al, 1995; Thompson and Thilaganathan, 1998; Chudleigh et al, 2001; Lee et al, 2006). This variability may significantly alter the incidence reported within the literature. Regardless, when an abnormality of the urinary tract is determined by antenatal ultrasonography several questions should be raised by the ultrasonographer and consulting urologist. Combinations of specific findings direct the differential diagnosis and permit a more accurate prognosis and tailoring of postnatal evaluation. The principal findings and their implications are listed in Table 114–1. Table 114–1 Elements of Prenatal Urologic Ultrasonographic Diagnosis ADPKD, autosomal dominant polycystic kidney disease; ARPKD, autosomal recessive polycystic kidney disease; MCDK, multicystic dysplastic kidney. Kidneys should be appropriate size for gestational age and relatively symmetrical (Chitty and Altman, 2003). Large differences in size may indicate contralateral compensatory growth. Absence of the kidney in the normal location may represent ectopia or agenesis/dysplasia. The normal kidney should be elliptical and have distinctive internal echolucency, representative of normal medullary pyramids (Fig. 114–1). The appearance of the medullary pyramids should not be confused with renal calyceal dilation. The echogenicity of the kidney should be slightly less than the corresponding spleen or liver. Abnormalities of echogenicity with or without hydronephrosis may indicate renal disease. Isolated increased echogenicity has been associated with renal parenchymal disorders, but it has also been shown to be of no clinical significance (Carr et al, 1995). When seen with hydronephrosis it may indicate renal dysplasia, particularly if there is accompanying decreased amniotic fluid (Kaefer et al, 1997b). Renal cystic disease can be seen in utero. Autosomal-recessive polycystic kidney disease (ARKPD) may appear as large, bright or echogenic kidneys, because the numerous small renal cysts cannot be resolved by ultrasonography. In contrast, a multicystic dysplastic kidney (MCDK) typically presents as a large noncommunicating macrocyst (Fig. 114–2). A single cyst may represent a dilated renal calyx or diverticulum, an atypical MCDK, severe hydronephrosis, or a nonrenal structure. In addition to kidney-specific findings, ureteral dilation, bladder filling and emptying, bladder wall thickness, intravesical cystic structures, dilation of the posterior urethra (keyhole sign), urinoma, amount of amniotic fluid, intra-abdominal/pelvic mass, and external genitalia should be noted. Hydroureter is best seen in cross section of a full bladder but is often difficult to detect (Fig. 114–3). Duplication and ureteral ectopia may also make this a difficult diagnosis. Although the bladder is sometimes difficult to image well, visualization of the bladder can be very informative, because a full bladder implies renal function. Inability to identify the bladder on repeat studies should raise the question of bladder exstrophy. Increased bladder wall thickness may indicate outlet obstruction, and dilation of the posterior urethra (keyhole sign) is strongly suggestive of posterior urethral valves (Fig. 114–4). In duplex systems, ureteroceles may present as an intravesical cystic structure and a dilated upper pole. Perirenal urinoma may indicate an obstructive condition (Yerkes et al, 2001) (Fig. 114–5). Typically it will have the appearance of an anechoic structure around the kidney or be in a subcapsular location. When identified, a urinoma or urinary ascites is often associated with cases of severe bladder obstruction, or posterior urethral valves, in which the urinoma may indicate a popoff mechanism. The popoff mechanism may be renal protective (Adzick et al, 1985). A urinoma also may be associated with a unilateral hydronephrotic/obstructed kidney (Mandell et al, 1994). Critical to the evaluation of the urinary tract is the assessment of the amniotic fluid level and changes during pregnancy. After 16 weeks, amniotic fluid production shifts from placental transudate to fetal urine; and by 20 to 22 weeks the vast majority of amniotic fluid is fetal urine (Takeuchi et al, 1994). Consequently, reduced amniotic fluid or oligohydramnios identified after 18 to 20 weeks’ gestation may be a result of urinary tract obstruction or poor renal development (Stiller et al, 1988). Proper identification of the external genitalia also can be very valuable in cases of gender-specific diagnoses, such as posterior urethral valves. In cases of virilization (e.g., congenital adrenal hyperplasia), a clitoris may appear as a small phallus; therefore the presence of scrotal testes is critical for male gender assignment (Benacerraf et al, 1989; Bromley et al, 1994; Mandell et al, 1995). Megalourethra, or a dilated, elongated penile urethra, may be an isolated anomaly or associated with prune-belly syndrome (Dillon et al, 1994) (Fig. 114–6). A female fetus with bilateral renal obstruction and bladder outlet obstruction would suggest a cloacal anomaly (Cilento et al, 1994; Ohno et al, 2000; Taipale et al, 2004) (Fig. 114–7). Hydronephrosis, or dilation of the renal pelvis, is the most common urologic abnormality found on ultrasound evaluation. Numerous grading systems have been developed; however, there is no consensus on the best and most consistent method of reporting ANH (Fig. 114–8) (Fernbach et al, 1993). Measurement of the APD has been used widely, but there have been no formal studies to determine the interobserver and intraobserver reproducibility of ANH measurement. One of the disadvantages of the APD can be the failure to describe pelvic configuration, calyceal dilation, and the laterality of findings, which should be included. The APD can be affected by gestational age, hydration status of the mother, bladder hypertonicity, and degree of bladder distention. Because the dimensions of the renal pelvis may normally increase with gestational age, most investigators have adjusted threshold APD values for early and later gestational age. Unfortunately, a simple threshold APD value that separates normal from abnormal does not exist, because even severe cases of ANH have the potential to resolve without incident, whereas mild degrees of ANH have the potential to progress (Pates and Dashe, 2006). Varying the minimal APD threshold can significantly alter the specificity and sensitivity of APD as a measure of ANH and postnatal pathology. To date there is no consensus on the optimal APD threshold for determining the need for postnatal follow-up. An APD cutoff of 15 mm for determining obstruction yielded a postnatal sensitivity of 73% and specificity of 82% (Coplen et al, 2006). A late gestational age APD cutoff of 10 mm would detect aproximately 23% of abnormal kidneys, whereas a cutoff of 7 mm detected 68% (Ismaili et al, 2003). One large systematic review estimated that only 11.9% of total pathology presented with late gestational age APD less than 9 mm, whereas 39% of total pathology was noted at APD levels less than 15 mm (Lee et al, 2006). Nearly identical results have been demonstrated by other investigators (Wollenberg et al, 2005). What appears certain is that lower cutoffs will be more sensitive in detecting postnatal pathology but will incur a higher false-positive rate. There is near agreement that APD greater than 15 mm represents severe or significant hydronephrosis, and some would also agree that a lower threshold of 4 to 5 mm is an appropriate value for considering APD to be abnormal (Feldman et al, 2001; Ahmad and Green, 2005; Wollenberg et al, 2005; Lee et al, 2006; Coelho et al, 2007, 2008). Taking these limitations into consideration, we define ANH in the second and third trimesters using APD thresholds for which the best available evidence provides prognostic information; along with these definitions the estimated distribution of the ANH based on the previous defined definition of APD is outlined in Table 114–2. As both ultrasound and MRI technology improve, more accurate radiographic information is obtainable (Laifer-Narin et al, 2007). However, determining accurate postnatal diagnosis and prognosis remains challenging. Regardless of the diagnosis, early intervention is uncommon except in the potential case of severe obstruction or posterior urethral valves. In the vast majority of other cases, early detection of ANH may be an impetus for future postnatal evaluation. The ability to determine definitive postnatal pathology based on antenatal findings is difficult. As an example, a recent systematic review of the ANH literature attempted to determine the risk of a pathologic diagnosis for patients with varying severity of ANH (Lee et al, 2006). The review included 1308 patients who were identified with ANH and sufficient postnatal radiographic follow-up. The degree of ANH was defined by APD identified in a particular trimester. Approximately 36% of the patients had a postnatal pathologic diagnosis, and the overall risk for any pathologic process increased with increasing degree of ANH (Table 114–3). However, the risk of vesicoureteral reflux (VUR) remained consistent regardless of the degree of ANH, thereby implying that ANH is not an appropriate indicator for VUR. Similarly, previous literature suggests that obstruction location can be determined prenatally in 88% of the cases; many others have reported a high false-positive rate (9% to 22%) (Hobbins et al, 1984; Scott and Renwick, 1993). The majority of false-positive findings in these studies were nonobstructive causes of hydronephrosis, such as high-grade reflux, large, nonobstructed extrarenal pelves, or transient hydronephrosis. Early and accurate diagnosis of posterior urethral valves is critical; however, it can be difficult. The hallmark signs of an in-utero diagnosis of posterior urethral valves have been described (e.g., oligohydramnios, dilated posterior urethra, thickened bladder, and hydroureteronephrosis). Other findings such as increased renal echogenicity and decreased amniotic fluid have also been suggested to be indicative of obstructive conditions (Kaefer et al, 1997b). Regardless, there are very few studies that have prospectively examined the clinical urologic implications of these findings alone or in combination (Lee et al, 2006). In one series of 22 fetuses, the false-positive rate was as high as 58% (Abbott et al, 1998), and in a population-based series the sensitivity in detecting valves was as low as 23% (Scott and Renwick, 1993). Regardless of the degree or severity of the finding, with any antenatal detection of a urinary tract anomaly a thorough fetal survey must be conducted. Amniocentesis and karyotype should be considered if intervention or a major anomaly is suspected, because the incidence of concurrent chromosomal anomalies is relatively high in fetuses with concomitant urologic anomalies (Callan et al, 1990; Nicolaides et al, 1992a, 1992b; Snijders et al, 1995). Key Points: Prenatal Diagnostic Findings The basic features of ureteropelvic junction (UPJ) obstruction in the fetus include dilation of the renal pelvis and collecting system with no evidence of ureteral dilation. Lee and associates (2006) demonstrated that increasing severity of ANH increased the likelihood of identifying postnatal UPJ obstruction. However, the threshold for recommending postnatal follow-up is largely arbitrary and currently there are no long-term prospective studies to determine the degree of postnatal evaluation, particularly for mild and moderate cases of ANH. Nevertheless, in the case of significant unilateral hydronephrosis there is little rationale for in-utero intervention or early delivery. In a few cases with massive dilation, therapeutic aspiration has been recommended for prevention of dystocia. In the case of bilateral UPJ obstruction the efficacy of in-utero intervention is difficult to assess. Severe forms of UPJ obstruction may be associated with urinary ascites or perinephric urinomas, which may be a predictor of nonfunction of the kidney (Mandell et al, 1994). The distinction between severe unilateral hydronephrosis and MCDK occasionally may be unclear. The findings of multiple noncommunicating cysts, minimal or absent renal parenchyma, and the absence of a central large cyst are diagnostic of MCDK (Bearman et al, 1976; Sanders and Hartman, 1984). The appearance of noncommunicating cysts is essential to the diagnosis and should be distinguished from severe hydronephrosis (see Fig. 114–2). Real-time examination of the kidneys to help determine the communication of the calyces is essential. Doppler ultrasonography of the renal vasculature pulse pattern has also been reported to help make the distinction (Kaminopetros et al, 1991). An MCDK may be present in any ectopic location but typically is in the normal position. In addition, MCDK can be present in a duplex kidney, typically the upper pole. If detected early in the pregnancy, the MCDK will involute over a period of time either prenatally or postnatally (Mandell et al, 1994). Bilaterally enlarged echogenic kidneys without renal cystic disease, particularly if associated with hepatobiliary dilatation or oligohydramnios, suggest ARPKD (Smedley and Bailey, 1987; Townsend et al, 1988) (Fig. 114–9). Typically this is identified before 20 weeks’ gestation, but later development has been reported (Zerres et al, 1988; Edwards and Baldinger, 1989; Mandell et al, 1991). Genetic testing is possible in some cases, thereby allowing for early diagnosis and the option for early termination because postnatal mortality and morbidity is high (Wilson, 2004; Zerres et al, 2004). A more challenging finding are normal-sized, diffusely echogenic kidneys that are not associated with other urologic lesions. A series of 19 cases (14 bilateral) included 10 patients with normal function who survived and 4 with ARPKD who died (Carr et al, 1995). Macrocystic disorders include the rare congential multilocular cystic nephroma, which is characterized by segmental involvement of the kidney with variable macrocysts (Eble and Bonsib, 1998); cystic Wilms tumor, which typically has larger amounts of functioning parenchyma; and ADPKD, which has heterogeneous cysts in size, location, and number (Reeders et al, 1986; McHugo et al, 1988; Ceccherini et al, 1989; Edwards and Baldinger, 1989; Novelli et al, 1989). Nonrenal cystic diseases are confused for MCDK; however, these cystic anomalies are typically not in the renal fossa and unlikely to be confused in the presence of two normal kidneys. These include mesenteric duplication cysts (Barr et al, 1990), neurenteric cysts (Uludag et al, 2001), bronchogenic cysts (Bagolan et al, 2000), extrathoracic pulmonary sequestration (Carpentieri et al, 2000), and cystic neuroblastoma (Granata et al, 2000). Duplication anomalies are often recognized on the basis of upper pole hydroureteronephrosis, associated with either an obstructing ureterocele within the bladder or ectopic ureter inserting outside the bladder (Vergani et al, 1999). Ureteroceles are identified as an intravesical, thin-walled cystic structure near the base of the bladder (Fig. 114–10). In cases of a very large ureterocele the ureterocele may be mistaken for the bladder. Alternatively, in the setting of an ureterocele the upper pole may not always present with hydronephrosis. The upper pole may appear as a cystic dysplastic unit without hydronephrosis and a concomitant ureterocele. This is often termed ureterocele disproportion (Share and Lebowitz, 1989). Additionally, single-system ureteroceles are more likely to occur in boys and have variable degrees of hydronephrosis of the entire kidney. Figure 114–10 Fetal ultrasonography showing an intravesical ureterocele. The ureterocele is indicated by the arrows and partially fills the bladder. With this finding the ultrasonographer should examine the upper tracts to determine whether there is hydronephrosis in the entire affected kidney or only the upper pole, as shown in Figure 114–3. Lower pole hydronephrosis may be present as a result of VUR (Fig. 114–11) or more rarely a lower pole UPJ obstruction. Occasionally, lower pole dilation is due to obstruction of both the upper and lower pole ureter by a large ureterocele. Similarly, bilateral hydronephrosis may be secondary to an element of bladder outlet obstruction from prolapse of the ureterocele into the bladder neck (Sozubir et al, 2003). In the absence of a ureterocele, upper pole hydroureteronephrosis suggests an obstructed ectopic ureter (Abuhamad et al, 1996). The dilated ectopic ureter can be mistaken for an ureterocele because of the impression that it creates on the back wall of the bladder. However, typically the ectopic ureter is much thicker than an ureterocele. Bilateral single-system ectopic ureters are uncommon but typically present as echogenic renal parenchyma, cystic disease, minimal bladder volume, and low amniotic fluid levels. Vesicoureteral reflux cannot be definitively diagnosed on prenatal ultrasonography, although intermittent or varying degrees of hydronephrosis or hydroureter are suggestive of the diagnosis. Several studies have demonstrated that a high incidence of VUR is associated with prenatally detected hydronephrosis; however, the true incidence of VUR in children with a history of ANH is difficult to determine based on the variability of postnatal diagnostic management in the literature (Lee et al, 2006). In two systemic reviews of the ANH literature a 10% to 15% incidence of VUR was identified regardless of the degree of ANH (Lee et al, 2006; van Eerde et al, 2007), indicating that the severity of ANH is not indicative of VUR and may not be the appropriate trigger for postnatal evaluation. In a neonate with prenatally detected hydronephrosis, the importance of diagnosing VUR remains controversial, because VUR diagnosed on postnatal evaluation for ANH is associated with earlier resolution of VUR (Estrada et al, 2009a). Perhaps the most important diagnosis to be made prenatally is that of posterior urethral valves in the male fetus. The finding of posterior urethral valves, at the very least, mandates prompt postnatal intervention, and, in some cases, prenatal intervention may be warranted. Fetal ultrasound findings include bilateral hydroureteronephrosis, a thick-walled bladder with dilated posterior urethra, and, in more severe cases, dysplastic renal parenchymal changes with perinephric urinomas and urinary ascites (see Fig. 114–5) (Bellinger et al, 1983; Reuter and Lebowitz, 1985; Barakat et al, 1991; Dinneen et al, 1993; Hutton et al, 1994; Gunn et al, 1995; Kaefer et al, 1997b; Abbott et al, 1998; Peters, 1998). Massive bladder dilation can be seen to occupy a good proportion of the abdomen (Fig. 114–12). With progression of the pregnancy, the bladder may become more thick walled with increasing posterior urethral dilation (see Fig. 114–4). When characteristic ultrasound findings are present the differential diagnosis includes prune-belly syndrome (with or without urethral atresia), massive VUR, and certain cloacal anomalies (in genetic females) (Kaefer et al, 1997a; Oliveira et al, 2000). Bladder exstrophy is a congenital abnormality affecting development of the lower abdominal wall, lower urinary and reproductive tracts, and musculoskeletal system. Prenatal diagnosis can be made with reasonable certainty using ultrasonography. Common observations in the fetus with bladder exstrophy include nonvisualization of the fetal bladder, a lower abdominal wall mass immediately inferior to a low-lying umbilicus, and diminutive genitalia (Gearhart et al, 1995). Patience and expertise in the ultrasonographer are important for recognition of the consistent negative finding on fetal imaging—absence of bladder filling—that is critical in making the diagnosis of bladder exstrophy. Other findings that may be evident to the experienced observer include normal kidneys in orthotopic position, normal vertebrae and spinal cord, abnormal symphyseal diastasis, and anteriorly displaced anus (Fig. 114–13). Prenatal diagnosis of bladder exstrophy provides an opportunity for discussion of bladder exstrophy, complex reconstruction as a neonate, follow-up care and outcomes, other organ system normality, and options of termination versus continuation of the pregnancy (Cacciari et al, 1999). The increasing ability to accurately diagnosis this and other complex diagnoses may have led to an increase in termination (Cromie et al, 2001).
Fetal Imaging
Fetal Diagnosis
PARAMETER
COMMENT
POSSIBLE CAUSES
Hydronephrosis
Variable severity; may include pelviectasis and/or caliectasis
Obstruction, reflux
Caliectasis
Intrarenal dilation; more indicative of significant pathologic process
Obstruction, reflux
Pelvic anteroposterior diameter
Measured in the coronal plane, variable; in extremes may predict clinical outcome; caution should be exercised in overreliance on these measurements
Increased in obstruction, reflux
Renal parenchyma
Echogenicity should be less than that of liver or spleen; lucent medullary pyramids should be seen
Increased echogenicity in dysplasia, obstruction, ARPKD
Urothelial thickening
Increased thickness of pelvic lining
Variable dilation as with reflux or occasionally obstruction
Duplication
Separation of renal pelvic sinus echoes when no hydronephrosis seen
Possible associated reflux or obstruction; look for dilated ureter and ureterocele
Cystic structures, renal
Simple cysts rare
MCDK, ADPKD
Cystic structures, intravesical
May be very large and fill bladder; thin walled
Ureterocele
Urinoma
Fluid collection around kidney; perinephric or subcapsular
Obstruction
Bladder filling
Fill and void cycles may be demonstrated over time
Urine production
Bladder wall thickness
Must be interpreted in context of bladder filling
Obstruction, neurogenic dysfunction
Keyhole sign
Dilated posterior urethral; difficult to image
Posterior urethral valves
Oligohydramnios
Markedly reduced amniotic fluid; usually considered as no pocket of fluid > 2 cm
Poor urine output because of obstruction and/or renal failure
Diagnostic Findings
Kidney
Ureter, Bladder, and Urinoma
Amniotic Fluid
External Genitalia
Hydronephrosis
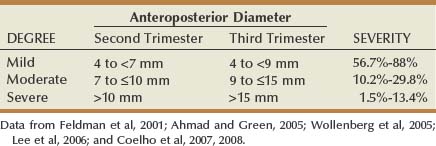
Categorizing ANH by APD
Diagnostic Accuracy
Specific Diagnoses
Ureteropelvic Junction Obstruction
Cystic Kidneys
Duplication Anomalies and Ureteroceles
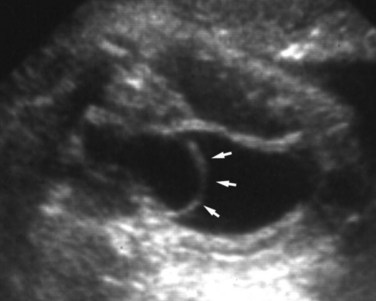
Vesicoureteral Reflux
Posterior Urethral Valves
Bladder Exstrophy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Perinatal Urology
• Ultrasonography is the mainstay of prenatal imaging. Selective use of fetal MRI may further delineate anatomic details and help with diagnosis and management.
• Inability to identify the bladder on repeat studies should raise the question of bladder exstrophy.
• The APD fails to describe pelvic configuration, calyceal dilation, and the laterality of findings.