Penis and Scrotum
ELSA F. VELAZQUEZ
MAHUL B. AMIN
ANTONIO L. CUBILLA
THE PENIS
Embryology
The genital eminence, an external mound arising between the umbilicus and the tail, is made up of the genital tubercle and the genital swellings.1 The urogenital sinus opens at the base of the genital tubercle, between the genital swellings. These structures form identically in male and female embryos up to 7 weeks’ gestational age. Development of the male external genitalia is dependent upon dihydrotestosterone, which is produced by the testes. At 9 weeks of gestational age, and under the influence of testosterone, the genital tubercle starts to lengthen. In addition, the genital swellings (also called the labioscrotal folds) enlarge and rotate posteriorly. As they meet, they begin to fuse from posterior to anterior. As the genital tubercle becomes longer, two sets of tissue folds develop on its ventral surface on either side of a developing trough, the urethral groove. The more medial endodermal folds will fuse in the ventral midline to form the male urethra. The more lateral ectodermal folds will fuse over the developing urethra to form the penile shaft skin and the prepuce. As these two layers fuse from posterior to anterior, they leave behind a skin line: the median raphe. By 13 weeks, the urethra is almost complete. A ring of ectoderm forms just proximal to the developing glans penis. This skin advances over the corona glandis and eventually covers the glans entirely as the prepuce or foreskin. The tip of the penis, which is now called the glans, then begins to form a cord of ectoderm that grows toward the spongy urethra. This cord is known as the urethral plate and when it canalizes, the end of the urethra (external urethral orifice) is at the tip of the penis.
The foreskin is formed in the 12th week of development. A septum of ectoderm moves inward around the edges of the penis and then breaks down, leaving a thin layer of skin surrounding the penis. During this time, the penis is also developing its corpora cavernosa and spongiosa from proliferating mesenchyme within the genital tubercle.
Anatomical Features
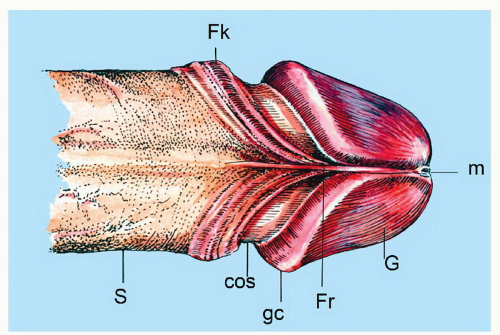
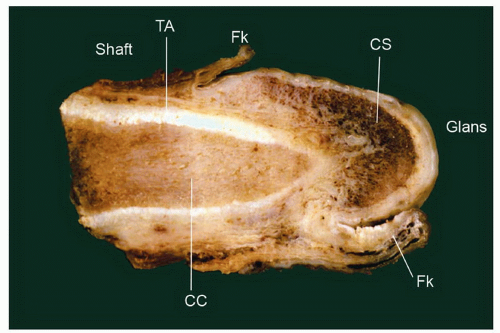
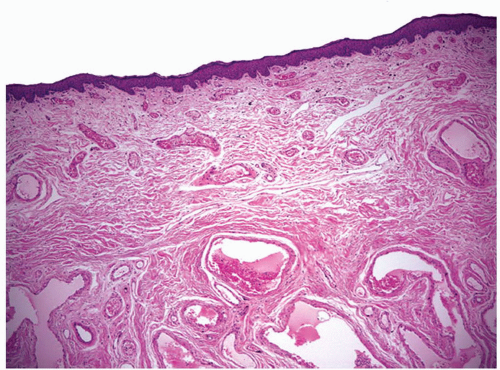
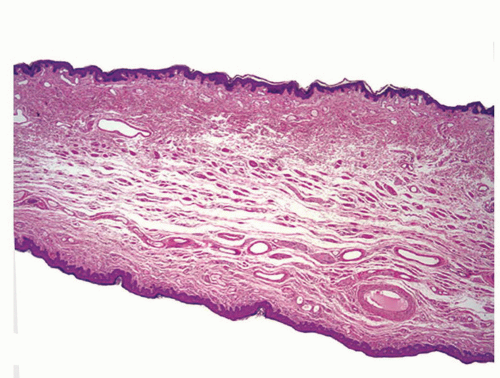
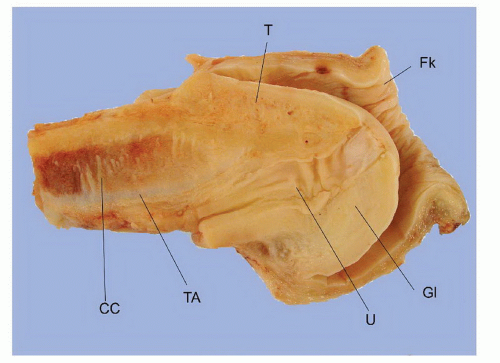
The complexity of the penis is partially related to the characteristics of the surface epithelium, which includes both skin and mucous membrane. The penis is composed of three portions: the distal part (glans or head), mid part (corpus or shaft), and proximal part (root) (Figs. 14-1 and 14-2).2,3 The head consists of the glans, coronal sulcus, and foreskin. The cutaneous portions of the penis include the root, shaft, and outer foreskin. The mucosal portions are the glans, coronal sulcus, and inner surface of the foreskin. The mucosal (distal) portion of the penis is particularly important from the surgical pathology viewpoint because it is from this portion that most squamous cell carcinomas (the most frequent malignancy at this site) arise. The foreskin or prepuce is a virtual sac that encases the head of the penis and distally reflects over the preputial orifice (Figs. 14-2 and 14-3). The inner (mucosal) surface of the prepuce is smooth and pink and the outer (cutaneous) surface is wrinkled and dark.3
The rubbery and conical glans contains the meatus urethralis, corona, and frenulum (Fig. 14-1). The meatus is vertical and is located at the apex, from which the frenulum arises, traversing the ventral portion of the glans to its insertion at the base where the glans is attached to the foreskin. The glans corona is a circumferential elevated rim at the base of the glans (Fig. 14-1). Grossly, the cut surface of the glans reveals four anatomical layers that from the surface to deep portions are a thin and white epithelium, lamina propria, corpus spongiosum, and corpora cavernosa (Figs. 14-2 and 14-3). The tunica albuginea is seen as a thick white sheath encasing the corpora cavernosa (Figs. 14-2 and 14-3). The coronal sulcus (balanopreputial sulcus) is a cul-de-sac located between the glans corona and foreskin. The preputial length is variable. In short foreskins, the preputial orifice is located proximally to the corona; in intermediate foreskins, the preputial orifice is located between the meatus and corona. Long foreskins entirely cover the glans. There is a significant association between long and phimotic foreskins and penile cancer.3
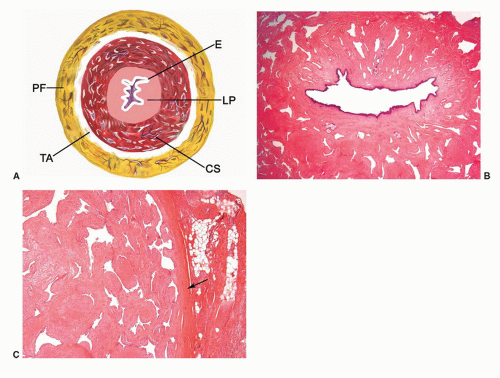
The main components of the shaft are the erectile tissues of the corpora cavernosa. A transverse cut section of the shaft
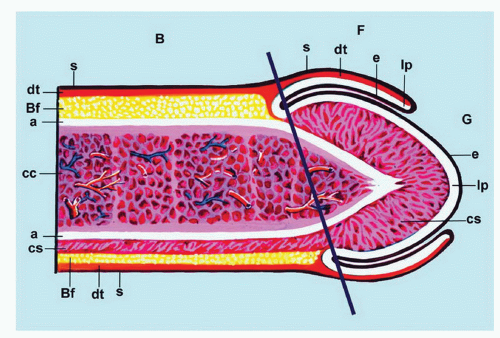
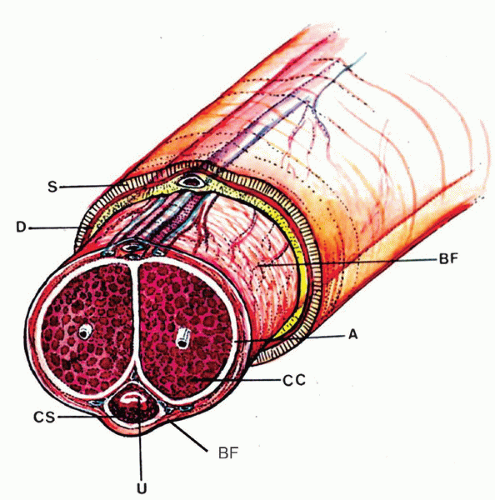
reveals, from outside to inside, the skin, dartos, penile fascia (Buck fascia), albuginea, erectile tissue (corpus spongiosum and corpora cavernosa), and urethra (Fig. 14-4).3 The penile or pendulous urethra is ventrally located in the corpus and head and runs surrounded by the corpus spongiosum (Fig. 14-4).
reveals, from outside to inside, the skin, dartos, penile fascia (Buck fascia), albuginea, erectile tissue (corpus spongiosum and corpora cavernosa), and urethra (Fig. 14-4).3 The penile or pendulous urethra is ventrally located in the corpus and head and runs surrounded by the corpus spongiosum (Fig. 14-4).
Microscopic Features
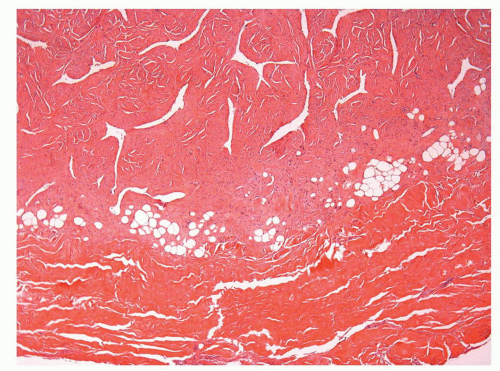
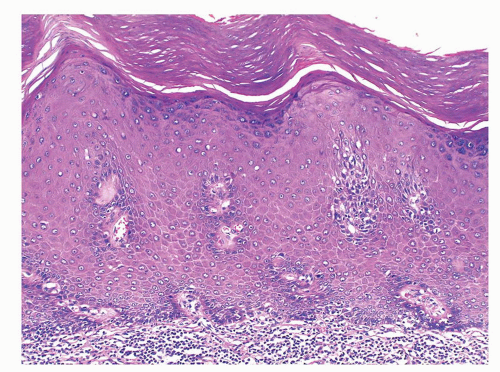
The glans is covered by a nonkeratinizing to slightly keratinized squamous epithelium without adnexal structures. The lamina propria, which measures 2 to 3 mm in thickness, is composed of loose connective tissue containing small blood and lymphatic vessels and nerves (Fig. 14-5). The corpus spongiosum is a complex erectile tissue predominantly composed of interanastomosed vascular channels separated by fibrous trabeculae. The thickness of the glans corpus spongiosum varies from 6 to 13 mm.3,8 The corpora cavernosa slightly protrudes into the glans in more than two-thirds of the specimens (Fig. 14-2).8 The penile shaft is predominantly formed by the erectile tissues of the thick corpora cavernosa and the thinner corpus spongiosum, the latter surrounding the urethra (Figs. 14-6 and 14-7).8 The corpora cavernosa are predominantly composed of thick and interanastomosing erectile vascular structures separated by a complex tridimensional
network of trabecula (Fig. 14-7). The vascular structures of the corpora cavernosa are thicker and more complex, and the connective tissue of the trabecula contains more smooth muscle bundles when compared with the corpus spongiosum.3 There are also thin nutritional vessels within the matrix of the corpora cavernosa. The tunica albuginea is a hyaline fibrous sheath measuring approximately 1 to 3 mm in thickness that surrounds and separates the corpus spongiosum and corpora cavernosa (Figs. 14-4, 14-6C, and 14-7). The Buck fascia surrounds the tunica albuginea and erectile tissues of the shaft and extends distally to the coronal sulcus where it is continuous imperceptibly with the connective tissue of the dartos or with the lamina propria (Figs. 14-4 and 14-6C). The Buck fascia is an important pathway of tumor progression and is composed of loose fibroadipose tissue with numerous blood vessels and nerves (Fig. 14-6C). It is surrounded by the dartos of the shaft that is present underneath the skin surface (Fig. 14-4).
network of trabecula (Fig. 14-7). The vascular structures of the corpora cavernosa are thicker and more complex, and the connective tissue of the trabecula contains more smooth muscle bundles when compared with the corpus spongiosum.3 There are also thin nutritional vessels within the matrix of the corpora cavernosa. The tunica albuginea is a hyaline fibrous sheath measuring approximately 1 to 3 mm in thickness that surrounds and separates the corpus spongiosum and corpora cavernosa (Figs. 14-4, 14-6C, and 14-7). The Buck fascia surrounds the tunica albuginea and erectile tissues of the shaft and extends distally to the coronal sulcus where it is continuous imperceptibly with the connective tissue of the dartos or with the lamina propria (Figs. 14-4 and 14-6C). The Buck fascia is an important pathway of tumor progression and is composed of loose fibroadipose tissue with numerous blood vessels and nerves (Fig. 14-6C). It is surrounded by the dartos of the shaft that is present underneath the skin surface (Fig. 14-4).
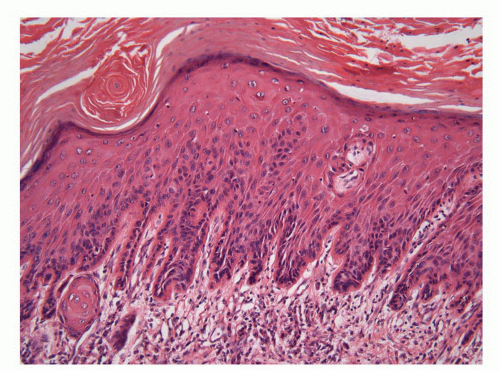
The foreskin shows five histologic layers that, from the inner to the outer surface, are the mucosal squamous epithelium, lamina propria, dartos, dermis, and epidermis (Fig. 14-8). The mucosal epithelium is nonkeratinizing to slightly keranized squamous, similar to that of the glans. Skin adnexa are absent; however, scarce, small sebaceous glands not associated with hair follicles may be rarely identified, especially near the cutaneous transition. The preputial lamina propria is thin and composed of loose fibrous tissue containing small vessels and nerves. Numerous genital corpuscles
are present in the superficial lamina propria just underneath the epithelium.6 The dartos is a muscular layer, in which irregularly arranged bundles of smooth muscle are seen in a background of loose connective tissue associated with vascular structures, nerves, and pacinian corpuscles.3 The skin is composed of the epidermis with a slightly hyperpigmented basal layer and a few small adnexal structures in the dermis.
are present in the superficial lamina propria just underneath the epithelium.6 The dartos is a muscular layer, in which irregularly arranged bundles of smooth muscle are seen in a background of loose connective tissue associated with vascular structures, nerves, and pacinian corpuscles.3 The skin is composed of the epidermis with a slightly hyperpigmented basal layer and a few small adnexal structures in the dermis.
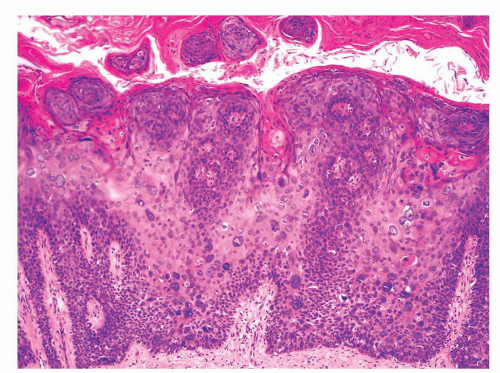
The anatomical levels in the coronal sulcus vary from three to four and from top to bottom include the squamous epithelium, lamina propria, dartos, and Buck fascia.3 The dartos is not present at this site in approximately half of the specimens. When present, it is continuous with the corporal and preputial dartos. The squamous epithelium is often keratinized but not associated with adnexal structures. The lamina propria is identical to that seen in the glans.
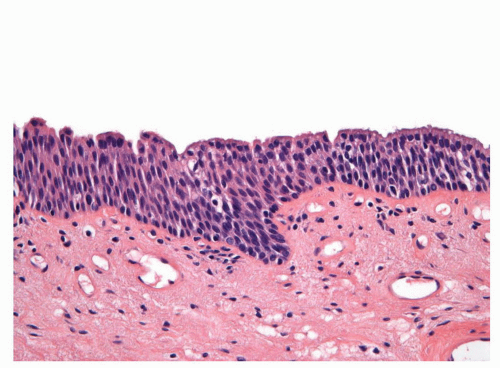 FIGURE 14-9 ▪ Histologic features of the penile urethral epithelium composed of stratified basaloid cells with a columnar cell layer at the surface. |
The penile urethral epithelium is composed of stratified basaloid cells with a columnar cell layer at the surface (Fig. 14-9). This epithelium differs from classical urothelium; umbrella cells are not present.4,9 Periurethral mucinous Littre glands as well as prostate-specific antigen-positive prostatic-like glands and ducts are associated with the urethra.10 The distal 5 to 6 mm of the penile urethra, which includes the fossa navicularis, shows a nonkeratinizing clear cell/hyperglycogenated squamous epithelium that is continuous with the glans epithelium.
Arteries
The penile arteries are branches of the internal pudenda, a branch of the iliac artery. They are divided in the dorsal and the cavernous systems. The dorsal arteries run from the base of the penis near and on both sides of the dorsal profunda vein within the Buck fascia and in the superior groove between the corpora cavernosa (Fig. 14-4). Smaller branches, the circumflex arteries, irrigate the corpora cavernosa and the periurethral corpus spongiosum. Terminal branches irrigate the glans, and collateral branches irrigate the skin. The cavernous arteries penetrate the corpora cavernosa at the site where the corpora join, and they run longitudinally near the central septum, which divides the corpora. The vasa vasorum, small arteries that irrigate the erectile tissues and the helicine branches, responsible for filling the vascular spaces during erection, originate from the cavernous arteries.13
Veins
The superficial dorsal vein drains the prepuce and skin and runs straight from the foreskin to the base of the penis located in the space between the dermis and Buck fascia. It opens into the superficial external pudendal vein. The deep dorsal
vein runs along the superficial dorsal vein but in a deeper plane beneath the Buck fascia. It receives the blood from the glans and corpora cavernosa and courses backward in the middle line between the dorsal arteries. The deep dorsal vein divides into two branches that drain into the pudendal plexus. It is noteworthy that the cavernous venous system delays venous drainage and in doing so assists in maintaining erections.14 The deep dorsal vein of the penis has a connection with the vertebral veins; hence it is possible for metastases to make their way to the vertebrae or even to the skull and brain without going through the heart and lungs. Pyogenic organisms may be transported by the same route.2
vein runs along the superficial dorsal vein but in a deeper plane beneath the Buck fascia. It receives the blood from the glans and corpora cavernosa and courses backward in the middle line between the dorsal arteries. The deep dorsal vein divides into two branches that drain into the pudendal plexus. It is noteworthy that the cavernous venous system delays venous drainage and in doing so assists in maintaining erections.14 The deep dorsal vein of the penis has a connection with the vertebral veins; hence it is possible for metastases to make their way to the vertebrae or even to the skull and brain without going through the heart and lungs. Pyogenic organisms may be transported by the same route.2
Nerves
Lymphatics
The lymphatics of the foreskin spring from a network that covers its internal and external surfaces; they arise from the lateral aspect and converge dorsally with the skin of the shaft lymphatics to form 4 to 10 vessels that run toward the pubis; here they diverge to drain into the right and left superficial inguinal lymph nodes. The lymphatics draining the glans form a rich network that, beginning in the lamina propia, course toward the frenulum where they coalesce with two or three trunks from the distal urethra to form several collecting trunks following the coronal sulcus. A collar of lymphatics entirely surrounds the corona, forming two or three trunks that run along the dorsal surface of the penis deep to the fascia together with the deep dorsal vein. At the presymphyseal region they form a rich anastomosing plexus draining into superficial and deep inguinal lymph nodes. The male urethra has a dense plexus in the mucosa. The lymphatic capillaries are especially abundant around the fossa navicularis.2 The lymphatics of the urethra, corpus spongiosum, and corpora cavernosa run toward the ventral surface of the body of the penis, reaching the raphe and the dorsum, where they run with the dorsal vein ending in the superficial and deep inguinal lymph nodes.11,12
Regional Lymph Nodes
Inguinal nodes are the first and most frequent site of metastasis in penile carcinomas. The inguinal lymph nodes can be divided into superficial and deep. A horizontal line crossing the point where the saphenous vein enters the femoral vein marks the division of these two compartments. The superficial nodes (approximately 10 to 13) are located above the cribriform fascia.2,9,11 The sentinel node is usually part of the superficial group of nodes located at the superior inner quadrant.11 Deep nodes are few, and their lymphatic vessels drain into the pelvic (iliac) lymph nodes, located externally or medially along the major iliac vessels.11,12
CONGENITAL ANOMALIES
Penile Agenesis
Congenital absence of the penis (aphallia) is caused by developmental failure of the genital tubercle.16, 17, 18 This rare anomaly has an incidence of approximately 1 in 30 million population. The phallus is completely absent, including the corpora cavernosa and corpus spongiosum. It is usually associated with normal scrotum and undescended testes. The urethra opens at any point of the perineal midline from over the pubis to the anus or anterior wall of the rectum. These patients often have other associated genitourinary anomalies.
Microphallus (Penile Hypoplasia)
The terms microphallus or micropenis refers to a penis with a stretched length more than 2.5 standard deviations less than the mean for age. It is usually associated with a normal scrotum and small, undescended testes. Micropenis should be differentiated from other types of pseudomicropenis, particularly the buried penis in the obese infant and the penis concealed by an abnormal skin attachment. Micropenis may be associated with endocrine and nonendocrine conditions.17,18
Penile Duplication
Duplication of the penis (diphallia) is another rare anomaly that results from incomplete fusion of the genital tubercle.17,18 It may present in two distinct forms. In the most common form, the patient exhibits a bifid penis, which consists of two separated corpora cavernosa that are associated with two independent hemiglans. The second form, or true diphallia, is extremely rare and varies from duplication of the glans alone to duplication of the entire lower genitourinary tract. The urethral opening can be in its normal position or in a hypospadiac or epispadiac position. Associated anomalies of the gastrointestinal, genitourinary, and musculoskeletal systems are frequently present.
Webbed and Buried Penis
Webbed penis is a common congenital abnormality in which a web or fold of scrotal skin obscures the penoscrotal angle. In the buried (concealed) penis, the penile shaft is hidden below the surface of the prepubic skin (Fig. 14-10A). This condition usually occurs in obese children in whom the abundant prepubic fat covers the penis.17,18
Penile Torsion
In penile torsion, there is a rotational defect of the penile shaft. The rotation usually is to the left in a counterclockwise fashion. The urethral meatus is placed in an oblique position, and the median raphe makes a spiral curve from the base of
the penis to the meatus. The embryologic abnormality often is an isolated skin and dartos defect. Penile torsion may also be associated with hypospadias or hooded prepuce.17,18
the penis to the meatus. The embryologic abnormality often is an isolated skin and dartos defect. Penile torsion may also be associated with hypospadias or hooded prepuce.17,18
Lateral Penile Curvature
Congenital penile curvature is a rare deformity secondary to asymmetry of corpora cavernosa length. Hemihypertrophy of a corpus cavernosum and its accompanying thickened tunica albuginea, with or without contralateral concomitant hypoplasia (rudimentary corpus), are responsible for the lateral deviation in congenital curvature of the penis.17,18
Penoscrotal Transposition
Complete penoscrotal transposition is an uncommon condition in which the scrotum is located in a cephalad position with respect to the penis. A less severe form is a bifid scrotum, in which the two halves of the scrotum meet above the penis. It is a heterogeneous anomaly, and detection warrants careful clinical evaluation to rule out other major and life-threatening anomalies, especially of the urinary system, gastrointestinal tract, upper limbs, craniofacial region, and central nervous system.17,18
Epispadias
Epispadias is a rare type of malformation in which the urethra ends in an opening on the dorsal aspect of the penis.17,18 It is the partial form of a spectrum of failures of abdominal and pelvic fusion in the first months of embryogenesis. The opening of the urethra may occur on the dorsal aspect of the glans (glandular epispadias), between the pubic symphysis and the coronal sulcus (penile epispadias) and at the penopubic junction (penopubic hypospadias).17,18
Hypospadias
Hypospadias is a birth defect in which the urethral meatus is abnormally placed on the ventral aspect of the shaft.17, 18 The urethral opening may be seen at any point along the urethral groove from the glans to the perineum. The urethral meatus
opens on the glans (first degree hypospadias) in about 50% to 75% of cases. Second degree (when the urethra opens on the shaft) (Fig. 14-10B) and third degree (when the urethra opens on the perineum) occur in up to 20% and 30% of cases, respectively (Box 14-1). The more severe degrees are more likely to be associated with other malformations. Hypospadias is among the most common birth defects of the male genitalia.17,18
opens on the glans (first degree hypospadias) in about 50% to 75% of cases. Second degree (when the urethra opens on the shaft) (Fig. 14-10B) and third degree (when the urethra opens on the perineum) occur in up to 20% and 30% of cases, respectively (Box 14-1). The more severe degrees are more likely to be associated with other malformations. Hypospadias is among the most common birth defects of the male genitalia.17,18
Box 14-1 • HYPOSPADIAS
|
Chordee
This anomaly is ventral or rotational curvature of the penis, which is most apparent with erection and is caused by fibrous tissue along the usual course of the corpus spongiosum. It is often associated with hypospadias.
Urethral Meatal Stenosis
Most commonly acquired after newborn circumcision in boys, urethral meatal stenosis is occasionally congenital and associated with hypospadias. Meatotomy is needed for a significantly deflected stream or for a pinpoint stream.
Median Raphe Cysts (Genitoperineal Raphe)
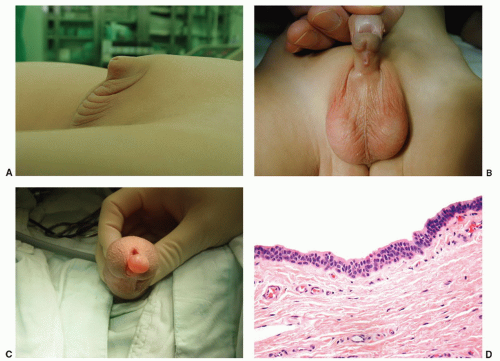
Median raphe cysts are unusual lesions that result from anomalies in the development of the urethral groove (ectopia of urethral and periurethral mucosa).18,19 They occur in the midline and ventral aspect of the penis, most frequently near the glans or prepuce, although they may be found anywhere from the urethral meatus (Fig. 14-10C) to the anus. They appear as a solitary, usually asymptomatic papule or nodule. Histologically, the epithelial lining has been described as squamous, pseudostratified columnar, stratified columnar, mucus-producing, ciliated, and apocrine (Fig. 14-10D).19,20
INFECTIONS
Balanitis is defined as inflammation of the glans penis, often involving the prepuce (balanoposthitis).
Bacterial Infections
Balanoposthitis is more frequently seen in uncircumcised men due to the greater propensity of pathogenetic bacteria to adhere to and colonize the mucosal surface of the foreskin.21, 22, 23 Several common Gram-positive bacteria may affect the genital skin; however, they are rarely biopsied. Balanoposthitis caused by Gardnerella vaginalis24 has rarely been reported. These infections usually are sexually transmitted. Histologically, a nonspecific inflammatory infiltrate is found. Gonococcal infections are typically sexually transmitted, but they more frequently produce urethritis. Gonococcal infections have been reported to infect penile median raphe as well.25
Cellulitis can result as a complication of localized infections especially in newborns and immunosuppressed patients. It is usually caused by group A streptococcus and less frequently by group B streptococcus and commonly involves the scrotum.26 Cellulitis is characterized by marked dermal edema with perivascular and interstitial predominantly neutrophilic infiltrate. This diagnosis needs confirmation by culture.
Trichomycosis pubis is an often asymptomatic colonization of the hair by various corynebacteria (especially Corynebacterium tenuis). It is characterized by a yellow, red, or black coating around pubic and/or scrotal hairs that under the microscope correspond to aggregates of Gram-positive bacteria adhering to the hair shaft (Fig. 14-70A and B).27,28 This condition is discussed in more detail in the scrotal section.
Fournier gangrene is a necrotizing fasciitis of the genitalia, perianal, and perineal regions that when affecting the penis (and scrotal skin) usually involves the dartos and Buck fascia.29,30 This condition particularly affects the scrotum and is discussed in more detail in the scrotal section.
Gangrenous balanitis (Corbus disease) is a rapidly progressing necrotizing infection, frequently caused by anaerobic organisms; it usually affects the glans penis, which sometimes may suffer complete necrosis.31 Necrotizing gangrene is an exceptional condition that may be seen in diabetic patients and associated with penile prosthesis.
Ecthyma gangrenosum (Pseudomonal cellulitis) is usually a complication of pseudomonal sepsis, and it is characterized by necrotizing bacterial vasculitis with thrombosis and secondary tissular necrosis and ulceration. Gram-negative bacteria can be found surrounding the blood vessels. This condition has been reported in drug abusers and neutropenic patients.21
Mycobacterial Infections
Penile mycobacterial infections are exceptionally rare with Mycobacterium tuberculosis being the most frequent species. Tuberculosis of the penis can present as a primary focus, a direct spread from nearby areas, or by hematogenous spread in generalized tuberculosis.32 Histologic features do not differ from tuberculous granulomas of other sites. BCG balanitis as a complication of intravesical BCG immunotherapy is extremely rare.33 A case of penile infection caused by Mycobacterium celatum in a man with acquired immunodeficiency syndrome has been reported.34
Syphilis
This sexually transmitted disease caused by the Treponema pallidum can manifest by anogenital lesions during the primary, secondary, tertiary, and congenital stages of the infection.21,35 The primary syphilis chancre occurs at the inoculation site 3 weeks after exposure to the spirochete; it
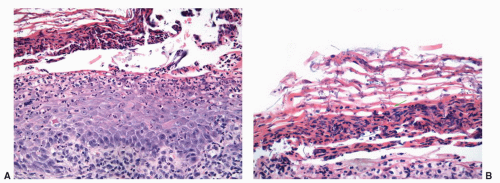
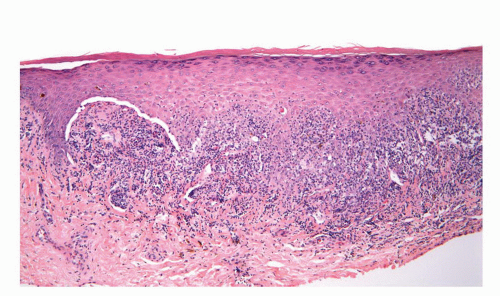
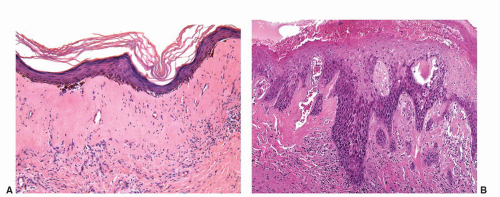
usually starts as a painless papule that enlarges and ulcerates centrally. This ulcerated lesion that has an indurated base is known as the hard chancre. Chancres are most often solitary and in the penis commonly affect the inner prepuce, coronal sulcus, penile shaft, and penile base.21 Secondary syphilis results from hematogenous dissemination of organisms. Condyloma lata, the characteristic anogenital lesions of secondary syphilis, are large verruciform papules, nodules, or plaques, which may become confluent.35 Gummas are characteristic of tertiary syphilis. Histologic hallmark of the lesions is obliterative endarteritis surrounded by a plasma cell-rich infiltrate (Fig. 14-11A and B). In primary syphilis the endarteritis can be found at the base of the ulcer. Secondary syphilis is usually associated with psoriasiform epidermal hyperplasia with a superficial lichenoid and deep perivascular plasma cell-rich infiltrate; the endarteritis can be superficial or deep.36, 37, 38 Spongiform pustular lesions can also be seen. The causative agent can be identified in primary and secondary lesions using Steiner or Warthin-Starry stains (Fig. 14-11C). The presence of T. pallidum in the tissues can also be detected by immunohistochemistry and by PCR. Condyloma latum is usually characterized by prominent epidermal hyperplasia and numerous neutrophils in the epidermis and cornified layer.36, 37, 38 When intraepidermal/intracorneal neutrophils are prominent, spirochetes tend to be easily identified within the epidermis (Fig. 14-11C). Gummas of tertiary syphilis are necrotizing granulomatous lesions associated with obliterative endarteritis. Treponema organisms usually cannot be demonstrated in these lesions by special stains. Reactivation of syphilis in patients infected with human immunodeficiency virus (HIV) is becoming more frequent39,40 and the affected patients may present with an atypical course.
usually starts as a painless papule that enlarges and ulcerates centrally. This ulcerated lesion that has an indurated base is known as the hard chancre. Chancres are most often solitary and in the penis commonly affect the inner prepuce, coronal sulcus, penile shaft, and penile base.21 Secondary syphilis results from hematogenous dissemination of organisms. Condyloma lata, the characteristic anogenital lesions of secondary syphilis, are large verruciform papules, nodules, or plaques, which may become confluent.35 Gummas are characteristic of tertiary syphilis. Histologic hallmark of the lesions is obliterative endarteritis surrounded by a plasma cell-rich infiltrate (Fig. 14-11A and B). In primary syphilis the endarteritis can be found at the base of the ulcer. Secondary syphilis is usually associated with psoriasiform epidermal hyperplasia with a superficial lichenoid and deep perivascular plasma cell-rich infiltrate; the endarteritis can be superficial or deep.36, 37, 38 Spongiform pustular lesions can also be seen. The causative agent can be identified in primary and secondary lesions using Steiner or Warthin-Starry stains (Fig. 14-11C). The presence of T. pallidum in the tissues can also be detected by immunohistochemistry and by PCR. Condyloma latum is usually characterized by prominent epidermal hyperplasia and numerous neutrophils in the epidermis and cornified layer.36, 37, 38 When intraepidermal/intracorneal neutrophils are prominent, spirochetes tend to be easily identified within the epidermis (Fig. 14-11C). Gummas of tertiary syphilis are necrotizing granulomatous lesions associated with obliterative endarteritis. Treponema organisms usually cannot be demonstrated in these lesions by special stains. Reactivation of syphilis in patients infected with human immunodeficiency virus (HIV) is becoming more frequent39,40 and the affected patients may present with an atypical course.
Chancroid
Chancroid or soft chancre is a sexually transmitted disease caused by a Gram-negative, facultative anaerobic bacterium, Haemophilus ducreyi.21,41,42 It is clinically characterized by a soft-based painful ulcer with yellow-gray base and sharp undermined border usually located in the coronal sulcus or glans. The ulcerated lesion can be small (dwarf chancroid) or rapidly enlarging and associated with ruptured inguinal abscess (giant chancroid). The combination of a painful
ulcer with tender adenopathy is suggestive of chancroid. Phagedenic chancroids are widely necrotic and destructive lesions usually secondary to superimposed infection by Fusobacterium organisms. Histologically, the soft chancre is characterized by a zonation phenomenon. The upper layer shows necrosis, fibrin, and numerous neutrophils; the middle layer shows abundant granulation tissue with prominent blood vessels, some with partial thrombosis; the deepest layer shows an intense plasma and lymphoid cell infiltrate.37,38,42 Diagnosis is made isolating H. ducreyi on special culture media.
ulcer with tender adenopathy is suggestive of chancroid. Phagedenic chancroids are widely necrotic and destructive lesions usually secondary to superimposed infection by Fusobacterium organisms. Histologically, the soft chancre is characterized by a zonation phenomenon. The upper layer shows necrosis, fibrin, and numerous neutrophils; the middle layer shows abundant granulation tissue with prominent blood vessels, some with partial thrombosis; the deepest layer shows an intense plasma and lymphoid cell infiltrate.37,38,42 Diagnosis is made isolating H. ducreyi on special culture media.
Granuloma Inguinale (Donovanosis)
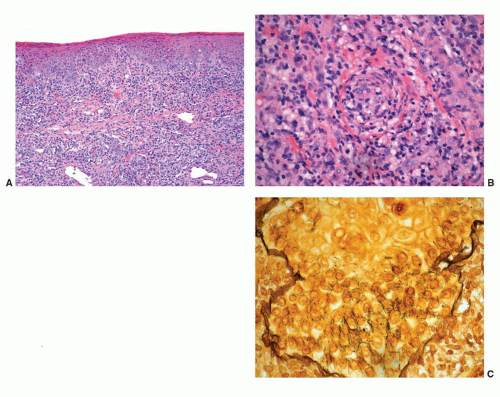
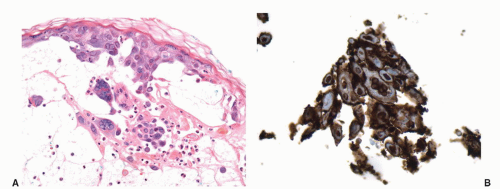
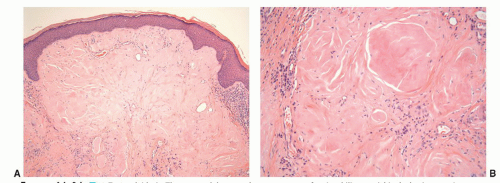
Granuloma inguinale is a sexually transmitted disease caused by the Gram-negative bacillus Calymmatobacterium granulomatis.21,43 The first clinical manifestation is a small, relatively painless nodule, occurring on the prepuce, glans, penile shaft, or scrotum that later ulcerates and may show large size. The ulcer has a red beefy base and hyperplastic borders. Histologically, the base of the ulcer shows exuberant granulation tissue with pseudoepitheliomatous hyperplasia at the borders (Fig. 14-12A). Numerous plasma cells and neutrophils are seen in the granulation tissue (Fig. 14-12B). A characteristic feature is the presence of large mononuclear foamy histiocytes with intracytoplasmatic Donovan bodies representing the microorganisms (Fig. 14-12B). The organisms are difficult to see with hematoxylin and eosin stain and can be highlighted with Warthin-Starry and Giemsa stains as short bacilli, either singly or in clumps (Fig. 14-12C).37,38,43 Electron microscopy reveals that the bacteria reside in phagosomes.43 Long-standing cases may be associated with elephantiasis of the penis and scrotum. Subcutaneous satellite lesions (pseudobuboes) can be seen. Lymph node involvement is rare.
Lymphogranuloma Venereum (Inguinale)
Lymphogranuloma venereum is a sexually transmitted disease caused by an obligate intracellular bacterium, Chlamydia trachomatis.21,37,38 Clinically, a painless papule or ulcer appears at the site of inoculation and then rapidly disappears. Within 1 to 2 weeks after the appearance of the primary lesion, enlargement of the inguinal lymph nodes begins (bubo formation). Histologically, the primary penile lesion shows nonspecific changes consisting of ulceration
and a nonspecific granulation tissue with plasma cells and lymphocytes. Nonnecrotizing granulomas composed of epithelioid histiocytes and a few giant cells surrounded by plasma cells also can be seen.37,44 The lymph nodes show focal aggregates of neutrophils in necrotic foci followed by follicular hyperplasia with massive plasma cell infiltration. The small suppurative foci eventually coalesce to form the classical stellate abscesses with surrounding epithelioid cells and multinucleated giant cells.45 Sinuses and tracts can develop. Old lesions are characterized by extensively fibrotic lymph nodes. The microorganisms cannot be seen by ordinary histologic stains. The diagnosis can be confirmed by cultures. Serology may be useful.44
and a nonspecific granulation tissue with plasma cells and lymphocytes. Nonnecrotizing granulomas composed of epithelioid histiocytes and a few giant cells surrounded by plasma cells also can be seen.37,44 The lymph nodes show focal aggregates of neutrophils in necrotic foci followed by follicular hyperplasia with massive plasma cell infiltration. The small suppurative foci eventually coalesce to form the classical stellate abscesses with surrounding epithelioid cells and multinucleated giant cells.45 Sinuses and tracts can develop. Old lesions are characterized by extensively fibrotic lymph nodes. The microorganisms cannot be seen by ordinary histologic stains. The diagnosis can be confirmed by cultures. Serology may be useful.44
Viral Infections
Human Papillomaviruses: Condyloma Acuminatum
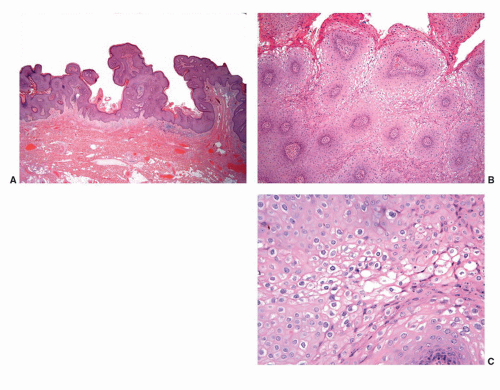
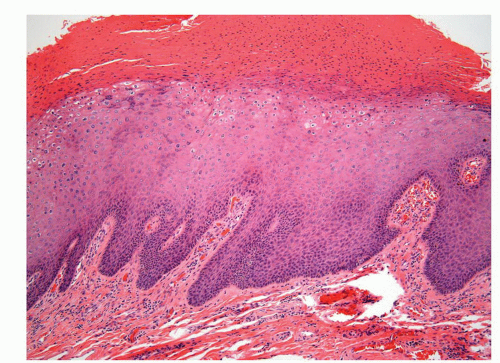
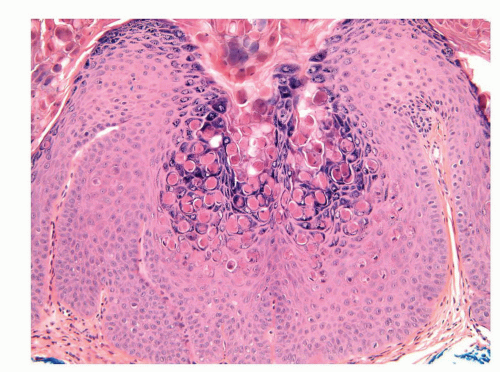
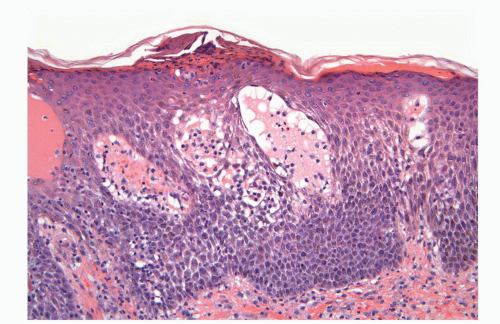
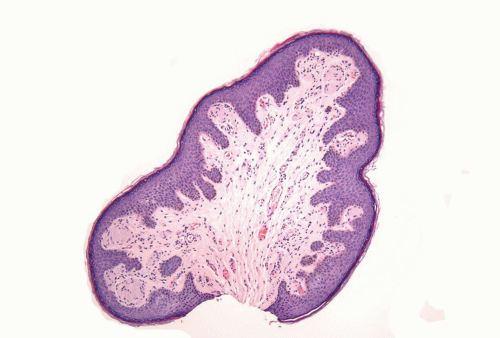
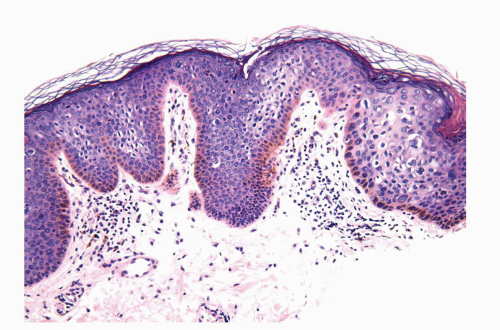
Human papillomaviruses (HPVs) are epitheliotropic DNA viruses that infect epithelial cells of the skin and anogenital and oropharyngeal mucosa. More than 100 genotypes have been described, several of which cause specific types of cancers and benign warts.45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 Genital wart (condyloma acuminatum) is one of the most common sexually transmitted diseases, affecting approximately 20 million people in the United States. HPV-6 and HPV-11, considered as low-risk types, are the ones most often associated with condyloma acuminatum.50 Condyloma acuminata tend to affect young adults and are often multiple. They may occur sporadically (usually associated with other genital infections) or in the setting of immunosuppression.51 They usually affect the glans, corona, frenulum, prepuce, and shaft and may extend into the meatus.21 They appear as soft, fleshy plaques with cobblestone or filiform appearance. Microscopically, classical lesions of condylomata acuminata are exophytic, branching, arborescent proliferations with acanthosis, hyperparakeratosis, and a fairly regular pushing, rounded base (Fig. 14-13A). The papillae frequently show a tree-like pattern with prominent central fibrovascular cores (Fig. 14-13A and B). The hallmark of the lesion is the presence of koilocytosis secondary to cytopathic viral effect (Fig. 14-13C).37,38 Koilocytes have enlarged, wrinkled nuclei surrounded by a perinuclear halo. Binucleated and multinucleated forms and dyskeratotic cells may be seen (Fig. 14-13C). These changes tend to be more prominent on the upper levels of the epithelium. The koilocytic changes are more difficult to detect in old lesions
that can look like fibroepithelial papillomas. Less frequently, condylomata may closely resemble seborrheic keratoses with basaloid cells, horn pseudocysts, and inconspicuous viral cytopathic changes (Fig. 14-14). Flat condylomata may also be seen (Fig. 14-15). Condyloma acuminatum is a benign lesion showing normal maturation with no atypias except for the koilocytosis that is usually restricted to the upper levels of the epithelium. Interestingly, it has been demonstrated that in genital locations lesions with features of fibroepithelial polyps and seborrheic keratosis without koilocytosis are often associated with HPV.57,58 Methods of virus identification include immunohistochemistry, in situ hybridization, and polymerase chain reaction.55,56,59,60
that can look like fibroepithelial papillomas. Less frequently, condylomata may closely resemble seborrheic keratoses with basaloid cells, horn pseudocysts, and inconspicuous viral cytopathic changes (Fig. 14-14). Flat condylomata may also be seen (Fig. 14-15). Condyloma acuminatum is a benign lesion showing normal maturation with no atypias except for the koilocytosis that is usually restricted to the upper levels of the epithelium. Interestingly, it has been demonstrated that in genital locations lesions with features of fibroepithelial polyps and seborrheic keratosis without koilocytosis are often associated with HPV.57,58 Methods of virus identification include immunohistochemistry, in situ hybridization, and polymerase chain reaction.55,56,59,60
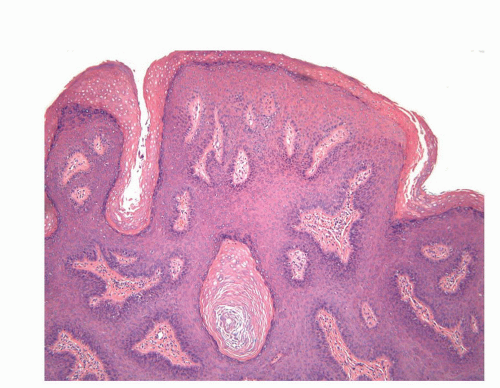 FIGURE 14-14 ▪ Old lesion of condyoma acuminatum mimicking a seborrheic keratosis. The koilocytic changes are more difficult to detect in such old lesions. |
It is important to differentiate condylomata previously treated with podophyllin from carcinoma. Treated condylomata may display prominent degenerative changes such as pallor of the epithelium, nuclear enlargement, necrotic keratinocytes, and increase in the number of mitotic figures (metaphase arrest).61 These degenerative changes tend to be focal and atypical mitoses should not be seen. Clinical correlation is necessary to make the correct diagnosis. True koilocytes should also be distinguished from the normal glycogenated keratinocytes of mucosal epithelia. Normal glycogenated keratinocytes appear vacuolated; however, they don’t show enlarged nuclei with irregular contours and are not binucleated.
Penile condylomata may reach large sizes (usually more than 8 cm), and after many years of neglect may become locally destructive (giant or atypical condyloma) or may harbor foci of evolving carcinoma.62 Due to the frequent association with squamous cell carcinoma, giant condylomata are discussed in the sections on squamous neoplasia. High-risk types of HPV are discussed in the section on squamous cell carcinoma.
Herpes
Herpes infection is caused by a DNA virus, the herpes simplex virus (HSV). Sexually transmitted genital herpes is primarily caused by HSV-2.21,63 Clinically, primary lesions present as multiple millimeter-sized fragile vesicles that rupture to form painful erosions and tend to be accompanied by lymphadenopathy. Recurrences tend to be less extensive. Penile lesions may affect the prepuce, shaft, and/or glans. Immunosuppressed patients may present with chronic herpetic ulcers. Histologically, lesions are characterized by intraepithelial vesicles containing prominent round acantholytic keratinocytes showing viral cytopathic changes. The latter consist of multinucleation, nuclear ground-glass appearance, and molding (Fig. 14-16A).37,38 Well-defined acidophilic inclusions can also be seen. The diagnosis can be made on Tzanck preparations. Herpes zoster can also affect the anogenital area and usually appears as grouped vesicles in a dermatomal distribution. Immunohistochemical analysis to identify HSV-1, HSV-2, and herpes zoster in paraffinembedded sections is now available (Fig. 14-16B).
Molluscum Contagiosum
This cutaneous DNA pox virus infection can be sexually transmitted. Lesions involving the anogenital region may also be secondary to autoinoculation, especially in children. Clinically, the lesions present as clustered, 3- to 6-mm domeshaped papules with central umbilication.21 Histologically, there are endophytic lobules of squamous epithelium separated by compressed dermis. Infected keratinocytes show the characteristic intracytoplasmic eosinophilic inclusions called Henderson bodies (Fig. 14-17).37,38 These inclusions are usually identified in the stratum spinosum and granulosum.
Penile Lesion in AIDS
HIV is a retrovirus of which two types, 1 and 2, are recognized. HIV-1 is the causative agent of AIDS in the United States and Europe. Immunodeficiency in AIDS is secondary to the depletion of T-helper cells as a result of HIV replication
selectively within those cells.21,37,38 The result of T helper cell depletion is a severe defect in cell-mediated immunity making the affected individuals particularly susceptible to infections. AIDS patients are often concomitantly infected with several different types of microorganisms. Skin diseases (including genital lesions) are common manifestations of HIV infection and they can be classified as noninfective dermatosis, infective disorders, and neoplasms.38,64, 65, 66, 67 These conditions are discussed in other parts of this chapter; however, they can be more frequent or severe in patients with AIDS. Immunosuppression often results in an atypical presentation, increased severity, and aggressive course of a dermatosis. Such lesions may also fail to respond to standard treatment regimens. A high index of suspicion is important to make the diagnosis.
selectively within those cells.21,37,38 The result of T helper cell depletion is a severe defect in cell-mediated immunity making the affected individuals particularly susceptible to infections. AIDS patients are often concomitantly infected with several different types of microorganisms. Skin diseases (including genital lesions) are common manifestations of HIV infection and they can be classified as noninfective dermatosis, infective disorders, and neoplasms.38,64, 65, 66, 67 These conditions are discussed in other parts of this chapter; however, they can be more frequent or severe in patients with AIDS. Immunosuppression often results in an atypical presentation, increased severity, and aggressive course of a dermatosis. Such lesions may also fail to respond to standard treatment regimens. A high index of suspicion is important to make the diagnosis.
Noninfective dermatosis includes seborrheic dermatitislike eruption, psoriasis, genital (aphthous ulcers), atopic dermatitis, and drug reactions. These conditions tend to be more widespread and severe in patients with AIDS. Histologically, the findings are similar (although they may be more florid) to those seen in patients without AIDS. A common finding in patients with AIDS is the presence of numerous plasma cells in the infiltrate.
Infective dermatosis includes a variety of bacterial, viral, and fungal disorders. Particularly common in AIDS patients are HPV-related lesions such as verruca vulgaris and condyloma accuminata that may be widespread and often associated with preneoplastic and neoplastic HPV-related tumors. Other common genital infections in AIDS patients include herpes viral infection (herpes simplex and herpes zoster), molluscum contagiosum, syphilis, Candida, and tinea cruris. Infestations such as scabies can be seen and often present as the Norwegian variant. Scraping of the lesions will show numerous mites.
Neoplastic conditions are also increased in AIDS patients. The most common tumors in such population include Kaposi sarcoma and HPV-related squamous cell carcinomas.66,67 Precursor lesions of squamous cell carcinoma (such as bowenoid papulosis and penile intraepithelial neoplasia [PeIN]) are also more frequently encountered.
Fungal Infections
Superficial Fungal Infections
Dermatophytosis
Dermatophytosis (tinea) is a superficial fungal infection caused by Trichophyton, Epidermophyton, and Microsporum species. Trichophyton rubrum and Trichophyton mentagrophytes are the most common agents.21,22 The fungus may infect the penis through local spread from more commonly affected areas such as the groin (tinea cruris). Patients may
also transfer the fungus from the feet or other areas by hand. Clinically, the lesion presents as an erythematous, often annular plaque with scaling. Histologically, the fungus may be difficult to identify on H&E-stained sections. Superficial fungal infections should be especially suspected when neutrophils are present in the squamous epithelium and keratin (often parakeratotic) layer. Special stains such as diastase-PAS and silver stains will highlight the presence of septate hyphae, frequently admixed with globose hyphal segments and chains of arthroconidia within the stratum corneum.22,37,38
also transfer the fungus from the feet or other areas by hand. Clinically, the lesion presents as an erythematous, often annular plaque with scaling. Histologically, the fungus may be difficult to identify on H&E-stained sections. Superficial fungal infections should be especially suspected when neutrophils are present in the squamous epithelium and keratin (often parakeratotic) layer. Special stains such as diastase-PAS and silver stains will highlight the presence of septate hyphae, frequently admixed with globose hyphal segments and chains of arthroconidia within the stratum corneum.22,37,38
Pytiriasis Versicolor (Tinea Versicolor)
This condition caused by the fungus Malassezia globosa may affect the penile shaft as hypo- or hyperpigmented macules.21,22,37,38 Unlike dermatophytes, the causative agent of pityriasis versicolor is usually easily identified on H&Estained sections as thin and basophilic septate hyphae and round yeasts (spaghetti and meatballs appearance). The fungus will also stain with PAS and silver stains. There is usually only a very mild inflammatory response within the dermis.
Candidiasis
Candidal balanoposthitis is the most common fungal infection of the penis. It is usually associated with a predisposing factor such as immunosuppression or diabetes mellitus. It may be sexually transmitted; however, approximately 15% to 20% of men may be asymptomatic carriers and this could explain some recurrent lesions. Primary lesions are characterized by papules and pustules that expand to form erosive plaques with small satellite pustules. Histologically, within the cornified layer and extending to the upper levels of the epithelium, there are long pseudohyphae and budding yeasts that stain with diastase-PAS and silver stains (Fig. 14-18A and B).21,22,37,38
Parasitic Infections
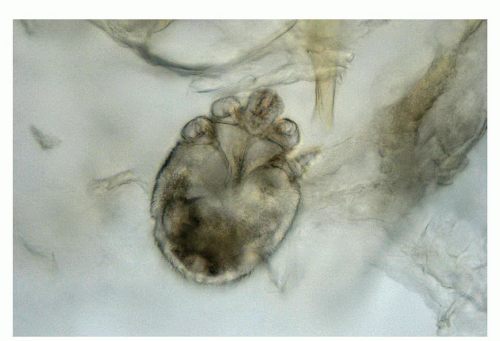
Several parasitic infestations including cutaneous larva migrans, schistosomiasis, amebiasis, and trichomoniasis have been described in the penis but are rare.21,70 The most common is scabies. This itchy infection is caused by the mite Sarcoptes scabiei, hominis variety, an obligate human parasite.21,37,38 The penis is commonly affected in the setting of a generalized infestation. The hallmark lesion is the burrow, which presents as a short, waxy, dark line. The lesions tend to be secondarily excoriated, eczematized, and impetiginized. Definitive diagnosis rests on microscopic identification of the mites, eggs, or pellets (scyballa). This is usually done by direct examination following scraping of the lesions or in shave or punch biopsies. On direct exam the mite measures about 400 μm in length and has a round to oval body (Fig. 14-19). On
histologic examination, there is usually a dermal lymphoid infiltrate with variable numbers of eosinophils. Several serial sections may need to be examined before identifying the burrow (which is almost entirely located within the cornified layer) and its contents (e.g., mite, eggs or fecal deposits).
histologic examination, there is usually a dermal lymphoid infiltrate with variable numbers of eosinophils. Several serial sections may need to be examined before identifying the burrow (which is almost entirely located within the cornified layer) and its contents (e.g., mite, eggs or fecal deposits).
NONINFECTIOUS INFLAMMATORY CONDITIONS
A variety of noninfectious dermatoses can preferentially or incidentally involve the penis and, especially in the latter case, can be problematic to diagnose.21,22,37,38 Examination of concomitant lesions from other sites (e.g., nongenital skin, oral mucosa, nails) can help to make the correct diagnosis. Dermatoses with a preferential and often exclusive genital location will be discussed below. Dermatoses that only incidentally affect the penis are too numerous; therefore, only the most frequent entities will be discussed.
Eczematous Dermatitis
Seborrheic dermatitis is the commonest form of eczema affecting this area and may be especially exuberant in patients with AIDS.21,22,64, 65 Other eczematous processes such as irritant contact dermatitis, allergic contact dermatitis, nummular dermatitis, and atopic dermatitis may all affect the penis. Allergic contact dermatitis may result from latex, lubricants, deodorant spray, and spermicides. More often, contact dermatitis is an irritant, resulting from persistent moisture and maceration. Clinically, the lesions may be prominent and atypical due to the highly vascularized and usually occluded nature of this area. Histologically, eczematous dermatitides are characterized by variable degree of epithelial hyperplasia, spongiosis, parakeratosis, and a superficial predominantly lymphoid infiltrate.37,38 Eosinophils may be numerous, especially in allergic contact dermatitis (Fig. 14-20). Seborrheic dermatitis, irritant contact dermatitis, and in general any of these dermatitides may be associated with neutrophils in the cornified layer and this is sometimes related to secondary impetiginization. In cases where aggregates of neutrophils are seen in the parakeratotic layer, one should consider the possibility of psoriasis and superficial fungal infection. A diastase-PAS stain is necessary to rule out the latter possibility.
Lichen Simplex Chronicus and Prurigo Nodularis
Lichen simplex chronicus (also known as circumscribed neurodermatitis) is characterized by the development of localized thickened scaly patches or plaques secondary to persistent scratching in patients who usually do not have an underlying dermatologic condition.21,38 Patients with atopic dermatitis and other eczematous processes may develop lesions of lichen simplex chronicus.
Lichen simplex chronicus preferentially affects the scrotum but the penis may also be involved. Histologically, there is epidermal hyperplasia with elongated rete ridges, orthohyperkeratosis often with patchy parakeratosis, a prominent granular layer, and variable but usually mild spongiosis.37 There is also papillary dermal fibrosis associated with a mild predominantly lymphoid infiltrate (Fig. 14-71). In some cases, nerve hyperplasia may be seen. The differential diagnosis includes psoriasis. Distinguishing features include irregularly thickened and long rete ridges associated with prominent granular layer and orthokeratosis in lichen simplex chronicus compared with the more uniform rete ridges and parakeratosis with diminished to absent granular layer in psoriasis. The characteristically fibrotic papillary dermis of lichen simplex chronicus is not a feature seen in psoriasis where usually there is a more edematous papillary dermis with prominent blood vessels.
Prurigo nodularis is characterized by intensely pruritic, chronic, lichenified nodules that are often excoriated. It shows a significant overlap and may be associated with lichen simplex chronicus. The histologic findings are similar to those seen in lichen simplex chronicus but usually the lesions are better circumscribed and the epidermal hyperplasia more prominent.
Prurigo nodule (Picker nodule) represents a solitary variant of prurigo nodularis.
Psoriasis
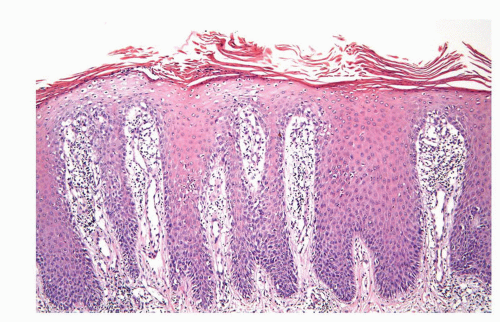
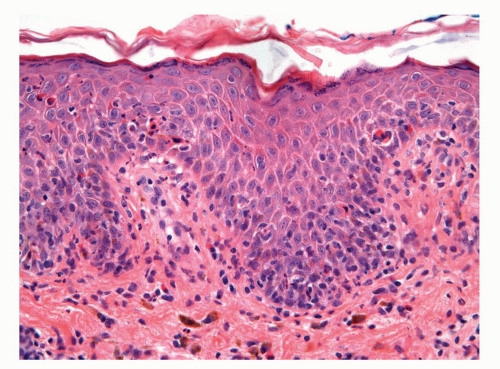
Psoriasis is a chronic relapsing and remitting common skin condition that may affect any site. It is one of the commonest of all skin diseases affecting approximately 2% of the US population.22,38 The classical cutaneous lesions are described as raised, sharply demarcated plaques with scaly surface. The clinical features, however, show regional variation. Lesions located on the penis may not show the classical features, and scaling may be minimal and moist and erosion-prominent.71 The clinical features may be mistaken by an eczematous process or even a preneoplastic condition (PeIN). Classical histopathologic features of well-established lesions of psoriasis include acanthosis showing evenly elongated rete ridges with club-shaped bases and characteristic
thinning of the suprapapillary plates. The papillary dermis appears edematous with prominent blood vessels.37,38 There is confluent parakeratosis containing neutrophilic aggregates and diminution to loss of granular layer (Fig. 14-21). Lesions in the genital location, however, may show more prominent spongiosis and even erosion. Therefore, often, the histologic features are not typical and the diagnosis is supported by the presence of classical psoriasis at other sites (scalp, nails, extensor surface, etc.). When dealing with a psoriasiform dermatitis containing neutrophils in the parakeratotic layer, a PAS stain to rule out superficial fungal infection is mandatory.
thinning of the suprapapillary plates. The papillary dermis appears edematous with prominent blood vessels.37,38 There is confluent parakeratosis containing neutrophilic aggregates and diminution to loss of granular layer (Fig. 14-21). Lesions in the genital location, however, may show more prominent spongiosis and even erosion. Therefore, often, the histologic features are not typical and the diagnosis is supported by the presence of classical psoriasis at other sites (scalp, nails, extensor surface, etc.). When dealing with a psoriasiform dermatitis containing neutrophils in the parakeratotic layer, a PAS stain to rule out superficial fungal infection is mandatory.
Reiter Syndrome
Reiter syndrome represents a triad of polyarthritis, urethritis, and nongonococcal conjunctivitis most commonly affecting males between 20 and 30 years of age.37,38,72 The pathogenesis is poorly understood but it appears that the condition develops in predisposed individuals usually following enteric or urogenital infections. Cutaneous and mucosal manifestations of the disease may be seen including a form of circinate balanitis presenting as a moist erosion affecting the glans and urethral meatus. Histologically, the lesions show a psoriasiform morphology with uniform elongation of rete ridges, parakeratosis, and edematous papillary dermis with prominent blood vessels. Neutrophils are numerous in the parakeratotic layer as well as in the epidermis, similar to what is seen in the pustular variant of psoriasis. A PAS stain should always be performed to rule out a fungal infection.
Lichen Planus
Lichen planus is a common, usually pruritic, and symmetrical papulosquamous dermatosis that affects the genital area in up to 40% of the patients with generalized lesions.21,38 Cases predominantly affecting the mucosal surface need to be distinguished from mucous membrane pemphigoid. The rare cases showing an exclusive genital or mucosal location may be difficult to diagnose. The classical lesions are violaceous, flat-topped shiny papules sometimes showing delicate white lines on the surface, namely, Wickham striae. Penile lesions tend to be associated with erosions. Histologically, lichen planus is characterized by a thickened or effaced epidermis often showing a saw-toothed appearance associated with a dense, band-like lymphohistiocytic infiltrate obscuring the dermal-epidermal junction where there is basal cell liquefactive degeneration and a variable number of cytoid (Civatte) bodies. When the lesions are located on the cutaneous surface, there is hyperorthokeratosis and typically wedge-shaped hypergranulosis (Fig. 14-22). Mucosal lesions tend to be associated with patchy hypergranulosis, parakeratosis, and a plasma cell-rich inflammatory infiltrate. Old lesions of lichen planus with only subtle interface changes and numerous plasma cells may be misdiagnosed as Zoon balanitis. Eosinophils are not a feature of lichen planus and when numerous, one should think of a lichenoid drug reaction. The features of lichen planus may overlap with lichen sclerosus and in some patients the two disorders may coexist.
Direct immunofluorescence studies in lichen planus may show fibrinogen and IgM along the basement membrane zone. Civatte bodies may also be highlighted, especially with IgM. Immunofluorescence helps to distinguish lichen planus from cicatricial pemphigoid.
Lichen Sclerosus (Et atrophicus) (Balanitis Xerotica Obliterans)
Lichen sclerosus is a chronic and atrophic mucocutaneous condition preferentially affecting anogenital areas of men and women. Extragenital location is less common. This condition
was described in the penis as balanitis xerotica obliterans by Stuhmer in 1928; however, because some authors prefer to use the term balanitis xerotica obliterans for the end-stage condition and to unify gynecologic and urologic terminology, the use of lichen sclerosus is recommended.75,76 Penile lichen sclerosus tends to affect middle-aged adults. Grossly, the lesions appear as white gray, irregular geographic and atrophic areas most commonly compromising the inner aspect of the foreskin, glans, and perimeatal region. Erosion, ulceration, and elevated hyperkeratotic foci may also be seen. In advanced cases, the preputial mucosal folds may disappear resulting in acquired phimosis or paraphimosis.21 Histologically, the lesions are characterized by an atrophic epithelium frequently intermixed with hyperplastic areas, vacuolar alteration of the basal layer, and a thickened lamina propria with the classical hyalinization/sclerosis (Fig. 14-23A).37,38 A variable amount of band-like lymphoid infiltrate is usually seen underneath the area of hyalinization. Because of marked basal cell vacuolar alteration, some cases may show dermal-epidermal clefting. Marked edema of the lamina propria may precede or coexist with the classical sclerotic changes.37,38 Lichen sclerosus is a superficial mucosal disorder preferentially affecting the epithelium and lamina propria and typically sparing the preputial dartos and corpus spongiosum of the glans. The lesions, however, tend to be broad and multifocal and may affect more than one epithelial compartment, and even extend to the epithelium and lamina propria of the distal urethra.76 While extragenital lichen sclerosus appears to carry no risk for malignant transformation, the relationship of anogenital lichen sclerosus and squamous cell carcinoma is well-documented.76, 77, 78, 79, 80, 81, 82 In a prospective study, the incidence of carcinoma arising in the setting of long-standing lichen sclerosus of the penis was 9.3%.80,81 In a retrospective review of 200 penectomy specimens with penile invasive carcinoma, 33% of the cases were associated with lichen sclerosus76 and this figure was much higher (69%) when considering carcinomas affecting the foreskin exclusively.82 When present adjacent to invasive carcinomas, lichen sclerosus is almost always associated with areas of epithelial hyperplasia and frequently shows squamous cell atypias (Fig. 14-23B).76,82 A significant association of lichen sclerosus with special (usually HPV-unrelated) variants of carcinoma such as usual, pseudohyperplastic, verrucous, and papillary carcinoma has been demonstrated. There is also a distinct association of lichen sclerosus with differentiated (simplex) PeIN.83 These findings suggest that lichen sclerosus may represent a precancerous condition for a subset of penile squamous cell carcinoma, especially the HPV-unrelated variants.
was described in the penis as balanitis xerotica obliterans by Stuhmer in 1928; however, because some authors prefer to use the term balanitis xerotica obliterans for the end-stage condition and to unify gynecologic and urologic terminology, the use of lichen sclerosus is recommended.75,76 Penile lichen sclerosus tends to affect middle-aged adults. Grossly, the lesions appear as white gray, irregular geographic and atrophic areas most commonly compromising the inner aspect of the foreskin, glans, and perimeatal region. Erosion, ulceration, and elevated hyperkeratotic foci may also be seen. In advanced cases, the preputial mucosal folds may disappear resulting in acquired phimosis or paraphimosis.21 Histologically, the lesions are characterized by an atrophic epithelium frequently intermixed with hyperplastic areas, vacuolar alteration of the basal layer, and a thickened lamina propria with the classical hyalinization/sclerosis (Fig. 14-23A).37,38 A variable amount of band-like lymphoid infiltrate is usually seen underneath the area of hyalinization. Because of marked basal cell vacuolar alteration, some cases may show dermal-epidermal clefting. Marked edema of the lamina propria may precede or coexist with the classical sclerotic changes.37,38 Lichen sclerosus is a superficial mucosal disorder preferentially affecting the epithelium and lamina propria and typically sparing the preputial dartos and corpus spongiosum of the glans. The lesions, however, tend to be broad and multifocal and may affect more than one epithelial compartment, and even extend to the epithelium and lamina propria of the distal urethra.76 While extragenital lichen sclerosus appears to carry no risk for malignant transformation, the relationship of anogenital lichen sclerosus and squamous cell carcinoma is well-documented.76, 77, 78, 79, 80, 81, 82 In a prospective study, the incidence of carcinoma arising in the setting of long-standing lichen sclerosus of the penis was 9.3%.80,81 In a retrospective review of 200 penectomy specimens with penile invasive carcinoma, 33% of the cases were associated with lichen sclerosus76 and this figure was much higher (69%) when considering carcinomas affecting the foreskin exclusively.82 When present adjacent to invasive carcinomas, lichen sclerosus is almost always associated with areas of epithelial hyperplasia and frequently shows squamous cell atypias (Fig. 14-23B).76,82 A significant association of lichen sclerosus with special (usually HPV-unrelated) variants of carcinoma such as usual, pseudohyperplastic, verrucous, and papillary carcinoma has been demonstrated. There is also a distinct association of lichen sclerosus with differentiated (simplex) PeIN.83 These findings suggest that lichen sclerosus may represent a precancerous condition for a subset of penile squamous cell carcinoma, especially the HPV-unrelated variants.
Balanitis Circumscripta Plasmacellularis (Zoon Balanitis)
This is an inflammatory condition of unknown etiology preferentially occurring in uncircumcised men.21,38,84,85 The classical clinical presentation is that of a solitary, reddish plaque with speckled and hemorrhagic surface usually affecting the glans. The clinical appearance usually raises the concern of a carcinoma in situ. Histologically, there is a thinned epidermis with no granular layer and a very thin to absent parakeratotic layer. There is some epidermal spongiosis and the keratinocytes are sometimes described as lozenge or diamond shaped.21 The classical (although nonspecific) feature that gave name to this entity is the presence of a dense, bandlike, plasma cell-rich dermal inflammatory infiltrate, usually associated with lymphocytes, extravasated erythrocytes, and siderophages. The blood vessels are prominent. The papillary dermal edema usually seen in early stage is replaced by fibrosis in long-standing lesions. The diagnosis of Zoon balanitis is largely one of exclusion. One must consider other specific entities such as lichen planus, syphilis, and pemphigoid
before making this diagnosis. Based on the nonspecific findings in Zoon balanitis and the similarities with other plasma cell-rich dermatitis and mucositis, the more generic term idiopathic lymphoplasmacellular mucositis-dermatitis was suggested by some authors to encompass etiologically uncertain lymphoplasmacellular infiltrates in the skin and mucosal surfaces.86
before making this diagnosis. Based on the nonspecific findings in Zoon balanitis and the similarities with other plasma cell-rich dermatitis and mucositis, the more generic term idiopathic lymphoplasmacellular mucositis-dermatitis was suggested by some authors to encompass etiologically uncertain lymphoplasmacellular infiltrates in the skin and mucosal surfaces.86
Fixed Drug Reaction
Fixed drug eruptions present as a one or more circumscribed erythematous to violaceous or brown plaques that show predilection for the extremities and external genitalia.21,38 Vesiculation and blister formation are common. Resolution is characterized by postinflammatory hyperpigmentation. The lesions classically recur at the same site each time that the patient is exposed to the same drug. The most common penile locations include the glans and distal shaft.21 Histopathologically, there is an interface dermatitis with prominent vacuolar degeneration of the basal layer, lymphocyte tagging of basal keratinocytes, and scattered apoptotic keratinocytes.37,38 These epidermal changes are associated with a superficial and midperivascular and interstitial mixed cell dermal infiltrate with lymphoid cells, eosinophils, and melanophages (Fig. 14-24). Old lesions may only show pigment incontinence. Fixed drug reactions can be distinguished from erythema multiforme by the deeper dermal infiltrate and presence of eosinophils.
Aphthous Ulcer and Behçet Disease
Aphthous ulcers may involve the scrotum and penis and they may or may not be associated with Behçet disease. Lesions are usually painful, solitary, and sharply marginated with necrotic gray fibrinoid base.21 Histologically, the changes are nonspecific and similar to those seen in oral aphthous lesions. At the center of the ulcer, there is necrosis with a dense neutrophilic infiltrate. At the periphery, there is a predominantly lymphoid infiltrate with exocytosis. Aphthous ulcer is a diagnosis of exclusion and should only be made after other causes (especially infection) have been ruled out. When chronic aphthous ulcers occur, the diagnosis of Behçet syndrome should be considered. Behçet disease is a poorly understand, chronic condition likely due to disturbances in the immune system. The presence of oral (apthtous) ulcers, along with any two out of the four additional signs: genital ulcers, skin lesions (e.g., pustules, erythema nodosum), eye lesions (e.g., iritis, uveitis) and pathergy reaction is necessary for the diagnosis.37
Bullous Pemphigoid
It is the most frequent autoimmune blistering disorder and usually affects the elderly.21,37 There are several variants that can variably affect the skin and mucosal surface. The characteristic lesion is a tense blister with an erythematous border. Approximately 7% of the patients have genital lesions. Histologically, well-established lesions show a subepithelial blister associated with edema of the underlying dermis or lamina propria and a mixed cell infiltrate containing numerous eosinophils. Direct immunofluorescence shows linear deposition of IgG and C3 at the basement zone. Two main bullous pemphigoid antigens are recognized: one is the intracellular protein 230 kD (BPAG1) and the other is the transmembrane protein 180 kD (BPSG2); both of them are located in the hemidesmosomal area of the basal keratinocyte.38
Cicatricial Pemphigoid (Mucous Membrane Pemphigoid)
Cicatricial pemphigoid is an autoimmune bullous disease that has a predilection for mucous membranes and often results in scarring. Oral and ocular lesions predominate; however, the genital area may also be involved. In males, genital lesions usually affect the foreskin and glans.38 Longstanding lesions may lead to urethral stricture formation and
phimosis.38,87 Histologically, there is a subepithelial blister associated with a mixed inflammatory infiltrate containing lymphoid cells, eosinophils, neutrophils, and plasma cells. The lamina propria may be edematous or fibrotic. Apart from scarring fibrosis in old lesions, these changes are identical to those seen in bullous pemphigoid. The mucosal lesions are often eroded and ulcerated showing fibrosing-granulation tissue with no specific acute and chronic inflammation. Direct immunofluorescence findings on perilesional mucosa (the site of choice) are similar to those seen in bullous pemphigoid.
phimosis.38,87 Histologically, there is a subepithelial blister associated with a mixed inflammatory infiltrate containing lymphoid cells, eosinophils, neutrophils, and plasma cells. The lamina propria may be edematous or fibrotic. Apart from scarring fibrosis in old lesions, these changes are identical to those seen in bullous pemphigoid. The mucosal lesions are often eroded and ulcerated showing fibrosing-granulation tissue with no specific acute and chronic inflammation. Direct immunofluorescence findings on perilesional mucosa (the site of choice) are similar to those seen in bullous pemphigoid.
Pemphigus
Pemphigus refers to a group of chronic blistering conditions that develop as a consequence of autoantibodies against a variety of desmosomal proteins.21,37 Several variants exist, including pemphigus vulgaris, vegetans, erythematosus, and foliaceous. Penile involvement is rare but may be observed.88,89 The hallmark of pemphigus is the presence of acantholysis, which is located at different levels of the epidermis in the different variants. Pemphigus vulgaris, the most common variant, is characterized by suprabasilar acantholysis with intraepidermal blister formation. In pemphigus vegetans, there is also suprabasilar acantholysis but this is more subtle and associated with a hyperplastic epidermis with scattered neutrophilic and eosinophilic abscesses.38 Pemphigus foliaceous and erythematosus are characterized by superficial acantholysis involving the upper levels of the epidermis. Direct immunofluorescence shows intercellular deposition of IgG and often C3.
Paraneoplastic pemphigus is a distinct variant of pemphigus that may be associated with a variety of malignancies. The penis may rarely be affected.38,90 The pathologic features are variable and often consist of a combination of suprabasal acantholysis and interface changes.38 Direct immunofluorescence may be negative in a fourth of the cases.
Acantholytic Dermatosis of the Genitocrural Area
This condition appears to represent a distinct acantholytic eruption of the genital/perineal area that should be distinguished from other generalized blistering acantholytic and dyskeratotic disorders that may also involve the genitalia. Originally reported as a localized form of acantholysis and dyskeratosis affecting the vulvocrural region in females, this rare condition was more recently described confined to the genital and perineal areas in male patients.38,91 The etiopathogenesis is unknown and perhaps somehow related to the moist environment of this area. Clinically, the lesions are characterized by multiple discrete papules or macerated patches involving the penis, scrotum, inner thighs, and perineum. Histologically, the acantholytic dermatosis had features resembling both Hailey-Hailey disease and Darier disease. The lesions show hyperkeratosis, parakeratosis, acanthosis, and acantholysis sometimes associated with dyskeratosis. Warty dyskeratoma-like features and follicular involvement may also be seen. Typically, inflammation is minimal or absent.
MISCELLANEOUS CONDITIONS
Amyloidosis
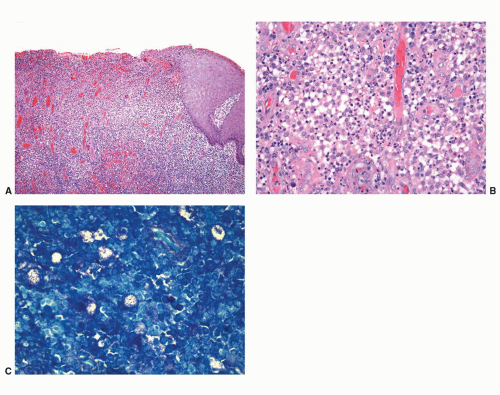
Penile amyloidosis is a rare disease that has been occasionally reported.92,93 It usually affects middle-aged men and presents as nodules or papules most commonly involving the glans. Coronal and shaft lesions may occur. Because of the nodular presentation, they may raise the clinical concern of a tumor. Penile amyloidosis is a localized disorder with excellent prognosis. It is usually limited to the skin/mucosa with rare systemic involvement. Histopathologically, nodular amorphous aggregates of eosinophilic material are identified in the dermis or lamina propria (Fig. 14-25). These deposits are positive with Congo red, crystal violet, and thioflavin T stain. Penile amyloidosis involving the skin or mucosa should be distinguished from urethral amyloidosis. Patients with urethral amyloid deposition most frequently present with hematuria, and the lesions are identified endoscopically.
Tancho Nodules and Paraffinomas
Tancho nodules refer to an unusual custom among some Asian populations to implant foreign material under the skin of the penis to improve sexual pleasure.94 Paraffinomas (also known as sclerosing lipogranulomas) result from the injection of mineral oil in the penis usually done with the purpose of penile enlargement. Histologically, these materials may cause foreign body reaction that may need surgical resection. Such foreign substances that are injected or inserted into the penis include paraffin, silicone, or wax. A characteristic foreign body reaction called paraffinoma similar to
the one seen in the scrotum is produced (Fig. 14-74).95 This reaction may occur several years after the injection.96
the one seen in the scrotum is produced (Fig. 14-74).95 This reaction may occur several years after the injection.96
Peyronie Disease
Peyronie disease is a relatively common condition (recent studies suggest that it may occur in up to 9% of the male population) that affects men in the third to seventh decade. It is characterized by dense fibrosis with formation of a plaque-like lesion affecting the tunica albuginea and penile fascia and sometimes extending to the dermis. This fibrotic plaque causes an abnormal penile curvature and very often pain and erectile dysfunction. In some cases, there are several firm nodules over the middorsal line. The histologic appearance varies with the duration of the disease. Early lesions are characterized by a loose proliferation of plum fibroblasts/myofibroblasta admixed with some inflammatory cells (similar to an early scar). Older lesions are more fibrotic and less cellular. Finally, large hypocellular nodules of hyalinized fibrotic tissue are the prominent finding. The changes in Peyronie disease recapitulate the sequence of events that characterize the development of tissue fibrosis in general. These are essentially an initial tissue insult (trauma, microtrauma, or local toxicity), followed by acute and then chronic inflammation that leads to deposition of excessive collagen and other extracellular matrix, fragmentation of elastin, and persistence of myofibroblasts. Fibrosis may then progress to partial ectopic calcification or ossification.97 An important experimental finding is increased levels of transforming growth factor β1 (TGFβ1), in the fibrotic plaques. Injury to the erect penis is thought to trigger the disease by inducing extravasation of fibrin and subsequent synthesis of TGFβ1. Some studies demonstrate a role for oxidative stress and cytokine release primarily TGFβ1 in the development of the fibrotic plaques.98,99 There is evidence indicating that these profibrotic factors interact with antifibrotic defense mechanisms, such as decrease of myofibroblast accumulation and elimination of reactive oxygen species by inducible nitric oxide synthase and neutralization of TGFβ1 by decorin, suggesting that some plaques are in dynamic turnover. Treatment is mainly surgical, as pharmacologic therapy has limited efficacy.98,99 The development of animal and cell culture models has advanced the understanding of this disease and uncovered several promising molecular targets for antifibrotic treatments.
Sclerosing Lymphangitis of the Penis
This unusual and usually self-limited condition preferentially affecting young adults is characterized by firm subcutaneous cord-like structures located along the dorsal shaft of the penis or around the coronal sulcus.100,101 The pathogenesis is poorly understood but it may be associated with trauma and vigorous sexual activity. Concurrent infections including herpes simplex infection have been described, but most likely they are not the cause of the disease. Because of the difficulty in distinguishing between large lymphatic vessels and veins in this location there is controversy as to whether this condition preferentially affects large lymphatic vessels or veins and perhaps either could be affected in different patients. This condition may show overlapping features with Mondor phlebitis. Histologically, one or more vessels of the superficial plexus show thickening of the wall, sometimes associated with thrombosis and various stages of recanalization. Inflammation is not prominent.
Vascular Disorders
Penile involvement by vascular disorders is rare. A few case reports of polyarteritis nodosa involving the penis can be found in the literature.102 Wegener granulomatosis affecting the penis has also been reported. Most reported patients developed penile lesions in the clinical context of upper respiratory tract, pulmonary, and/or renal involvement by the disorder.103
Crohn Disease
Anogenital lesions occur in about 30% of the patients with intestinal Crohn disease, either by direct extension of active intestinal disease or as noncontiguous lesions, or so-called metastatic disease. Skin involvement is more common in patients with colonic disorder. Importantly, skin lesions may be the first manifestation of the disease. Genital lesions in patients with Crohn disease include edema, ulcers, abscesses, sinus, and fistulas.37,38,104,105 Histopathologically, the findings are nonspecific and vary from edema and lymphangietasis to noncaseating granulomas that may have a perivascular distribution. Clinical correlation is important to confirm the diagnosis.
Papillomatosis of Glans Corona (Penile Pearly Papules)
This is an HPV-unrelated, asymptomatic, and benign condition occurring in 20% to 30% of normal men characterized by multiple pearly gray-white fibroepithelial papules located in the dorsal aspect of the glans corona.106 These minute papillomas are characteristically arranged in two to three rows. Each lesion is dome shaped or filiform and arises on a solitary base. Histologically, there is a normal or slightly thickened squamous epithelium overlying a central fibrovascular core (Fig. 14-26).
Penile Cutaneous Horn (Cornu Cutaneoum)
This is a clinical term based on the appearance of the lesion that presents as a keratotic protuberance mimicking a horn. Nonneoplastic and neoplastic conditions may present clinically as a cutaneous horn. The diagnosis rests upon histiologic examination of the viable epithelium underneath the thick cornified layer. Cutaneous horns may correspond to viral warts, seborrheic keratosis, or squamous cell carcinoma among other possibilities.37,38,107
Verruciform Xanthoma
The vast majority of these lesions affect the oral cavity (70%) followed by the anogenital region.21,38,108 Verruciform xanthoma usually affects adults and presents as a yellowish to red plaque. Most patients do not have associated lipid disorders. Histologically, most lesions shows acanthosis, papillomatosis, and a thick parakeratotic layer exhibiting a bright orange hue. Within the connective tissue core of the papillae and between rete ridges, there are numerous large, foamy, lipid-laden macrophages (Fig. 14-76). Multinucleated cells are usually not seen. Lymphocytes and neutrophils may be present. These lesions are nonrelated to HPV and should be differentiated from condylomata.
Phimosis
This is a condition in which the prepuce cannot be retracted, usually as a consequence of nonspecific chronic bacterial infections, lichen sclerosus, or congenitally abnormally long foreskin.21,109, 110 Phimosis may also be seen secondary to graft versus host disease. The accumulation of smegma induces a diffuse inflammation of all mucosal epithelial compartments of the glans and foreskin. The treatment of choice is the surgical removal of the foreskin. Histologically, there is usually fibrosis of the lamina propria associated with a nonspecific lymphoid and plasmacytic infiltrate.110 It is important for the surgical pathologist to liberally sample phimotic foreskin specimens from adults to rule out dysplasia, carcinoma in situ, or occult early invasive carcinoma. Special attention is advised to hyperkeratotic, thick, and slightly elevated or irregular foci. Penile cancer has been reported to occur more frequently in patients with long phimotic foreskins (Fig. 14-27).7 In paraphimosis, the prepuce cannot be advanced over the glans and becomes trapped in the space located between the coronal sulcus and the glans corona. Unusual cases of penile infarct secondary to arterial obstruction resulting from edema have been described.
Squamous Hyperplasia
Squamous hyperplasia is characterized by an acanthotic thickening of the squamous epithelium without atypia3,11,111,112 that may involve any of the penile anatomical compartments. It represents a reaction pattern more than a specific entity and it may be seen associated to a rather broad spectrum of conditions from inflammatory dermatitis (i.e., lichen sclerosus) to squamous cell carcinoma. Squamous hyperplasia is usually seen in the epithelium adjacent to special subtypes of carcinomas, particularly usual (keratinizing), verrucous, and lowgrade papillary variants. Grossly, lesions appear flat, smooth, and pearly white with occasional slightly elevated to papillary configuration. Microscopically, squamous hyperplasia shows acanthosis, hyper-/orthokeratosis, and normal maturation of squamous cells (Fig. 14-28). Patchy parakeratosis may occasionally be seen. Koilocytosis, deep keratin whorls, and cytologic atypia are not present. Pseudoepitheliomatous hyperplasia may be confused with squamous cell carcinoma (SCC) because the florid complex downward proliferation of squamous rete ridges may appear as detached from the epithelium in cut sections. Important features that may be helpful to differentiate pseudoepitheliomatous hyperplasia from carcinoma include the superficial nature of the lesion, absence of atypia, absence of deep keratin whorls (keratin pearls), and lack of stromal reaction or desmoplasia. Another pattern is that of verrucous hyperplasia, typically found adjacent to verrucous carcinomas (Fig. 14-28). The frequent association of squamous hyperplasia with differentiated PeIN and invasive carcinomas, especially low-grade variants and the usual continuity with the invasive tumor, suggests that squamous hyperplasia with or without lichen sclerosus may be a precancerous lesion of the penis.76 Its role as a potential precursor remains controversial.
SQUAMOUS NEOPLASIA
Penile Intraepithelial Neoplasia
Invasive squamous cell carcinomas are thought to be preceded by precursor lesions, namely, PeIN. In keeping with the notion of a bimodal pathway of carcinogenesis in penile carcinoma, precursor lesions can be broadly classified into two main groups: HPV-related and HPV-unrelated variants.83,111, 112, 113, 114, 115, 116, 117, 118 Taking into consideration the striking similarities in morphology and pathogenesis between vulvar and penile carcinomas,119, 120, 121 and in an attempt to have a simplified and more uniform terminology, we have recently proposed a slightly modified nomenclature for penile preinvasive lesions.83 The term PeIN is preferred over old terms such as squamous intraepithelial lesion (SIL), erythroplasia of Queyrat, and Bowen disease. These latter two terms are synonymous with carcinoma in situ and have been used for lesions in the glans (erythroplasia of Queyrat) and skin of the shaft (Bowen disease).122, 123, 124 PeIN can be classified as differentiated/simplex (HPV-unrelated) and undifferentiated (HPV-related) variants (Box 14-2). The latter can be subclassified as warty, basaloid, and mixed warty/basaloid.83 PeIN may be solitary or multifocal, and tends to be associated with infiltrating SCCs in about two-thirds of cases. In our experience of these cases, approximately 65% are associated with differentiated PeIN and 35% with warty/basaloid PeIN. Differentiated PeIN affects older patients, it usually arises in the setting of a chronic scarring inflammatory dermatosis, and it is more frequently located in the foreskin when compared with HPV-related variants. The latter affect younger patients and are usually more centrally located in the glans and perimeatal region. The gross appearance of PeIN is heterogeneous and does not allow one to distinguish between the two main types. Lesions vary from flat to slightly elevated, pearly white or moist erythematous, dark brown or black, macules, papules, or plaques. The contours may be sharp or subtle and irregular. Occasionally, a granular or low papillary appearance may be noted. Microscopically, differentiated (simplex) PeIN is characterized by a thickened epithelium, usually associated with elongated and anastomosing rete ridges, subtle abnormal maturation (enlarged keratinocytes with abundant eosinophilic cytoplasm), whorling and keratin pearl formation (usually in deep rete ridges), prominent intercellular bridges (spongiosis and sometimes acantholysis), and atypical basal cells with hyperchromatic nuclei.83,118,119 Parakeratosis is frequent (Fig. 14-29). At low power, the atypia seems to be present only in lower levels of the epidermis; however, at higher power, it is more clear that
there is subtle but abnormal maturation in all levels of the epithelium. Despite the subtle changes, we believe that differentiated PeIN represents a high-grade (although differentiated) lesion that may evolve to frank invasive carcinoma without showing more significant atypia (Fig. 14-30).83,118,119,121
there is subtle but abnormal maturation in all levels of the epithelium. Despite the subtle changes, we believe that differentiated PeIN represents a high-grade (although differentiated) lesion that may evolve to frank invasive carcinoma without showing more significant atypia (Fig. 14-30).83,118,119,121
Box 14-2 • PENILE INTRAEPITHELIAL NEOPLASIA
|
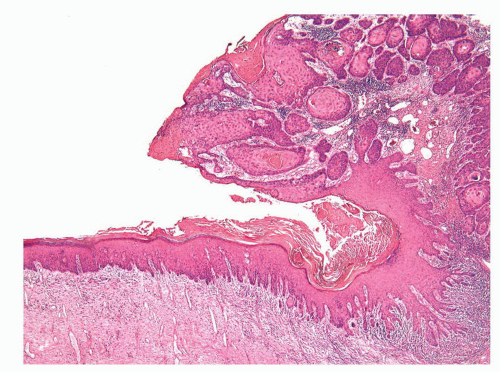 FIGURE 14-30 ▪ Differentiated PeIN (left) is seen adjacent to a well-differentiated invasive carcinoma (right). |
It is not surprising that the precursor lesions of welldifferentiated invasive tumors show such a high degree of differentiation. It is important to recognize this lesion because it appears to be the most frequent precursor lesion of penile carcinomas, especially the keratinizing and well-differentiated variants. Unfortunately, most studies on simplex PeIN are retrospective and have been done in penectomies for invasive carcinoma. It is also important to acknowledge the difficulty of this diagnosis and the need of molecular or immunohistochemical markers to more easily prospectively identify differentiated PeIN. p53 expression is more frequently seen in differentiated PeIN when compared with warty/basaloid variants112; however, p53 expression is also seen in benign condylomata and other inflammatory or reactive conditions and therefore cannot be considered a specific marker of differentiated PeIN. A preferential association was seen between lichen sclerosus and differentiated PeIN when compared with warty/basaloid variants.76,82,83 It is therefore important to keep a high level of suspicion when dealing with hyperkeratotic and hyperplastic lesions with subtle keratinocytic atypia arising in the setting of longstanding lichen sclerosus.
The second major type of PeIN (the undifferentiated, HPV-related type) shows distinctive morphologic changes. It is subclassified as warty, basaloid, and mixed PeIN. In the basaloid variant, the epithelium is replaced by a monotonous population of small immature cells with a high nuclear/cytoplasmic ratio (Fig. 14-31).83,118 Apoptosis and mitotic figures are numerous. Basaloid PeIN should be distinguished from transitional cell urethral carcinoma in situ, which may secondarily involve the penile meatal region.125 In the warty pattern, the involved epithelium has an undulating or spiking surface with atypical parakeratosis. There is striking cellular pleomorphism and koilocytosis (multinucleation, nuclei with irregular contours, perinuclear halo, and dyskeratosis) (Fig. 14-32). Mitotic figures tend to be numerous. Frequently, lesions show overlapping features of both, namely, mixed warty and basaloid PeIN. These mixed lesions tend to have a spiking surface with koilocytic changes while the lower half of the epithelium is predominantly composed of small basaloid cells (Fig. 14-33). Basaloid and warty PeIN can be divided into low-grade and high-grade lesions when the atypical cells occupy less than half and more than half of the epithelial thickness, respectively. Most of the warty and basaloid PeIN will fall within the high-grade category. Full-thickness atypia of the epithelium equals carcinoma in situ. Low-grade lesions are exceptional and should be distinguished from
benign condyloma, with the latter not being considered a preneoplastic condition. p16 is usually overexpressed in undifferentiated PeIN (Fig. 14-34) and negative in differentiated PeIN, further supporting the association of undifferentiated PeIN with high-risk variants of HPV.112 Other rare morphologic patterns of precursor lesions include pleomorphic, pagetoid, clear, spindle, and small cell; all of these are more likely to represent variants of the HPV-related group. A recent study found a distinctive geographical distribution of penile precursor lesions. PeIN with warty and/or basaloid features predominated in low-incidence areas, whereas differentiated PeIN was more prevalent in endemic regions for penile cancer. With few exceptions there is a good correlation between the microscopic appearance of the preinvasive process and the associated invasive carcinoma, further supporting the concept of a dual pathway of penile tumorigenesis.
benign condyloma, with the latter not being considered a preneoplastic condition. p16 is usually overexpressed in undifferentiated PeIN (Fig. 14-34) and negative in differentiated PeIN, further supporting the association of undifferentiated PeIN with high-risk variants of HPV.112 Other rare morphologic patterns of precursor lesions include pleomorphic, pagetoid, clear, spindle, and small cell; all of these are more likely to represent variants of the HPV-related group. A recent study found a distinctive geographical distribution of penile precursor lesions. PeIN with warty and/or basaloid features predominated in low-incidence areas, whereas differentiated PeIN was more prevalent in endemic regions for penile cancer. With few exceptions there is a good correlation between the microscopic appearance of the preinvasive process and the associated invasive carcinoma, further supporting the concept of a dual pathway of penile tumorigenesis.
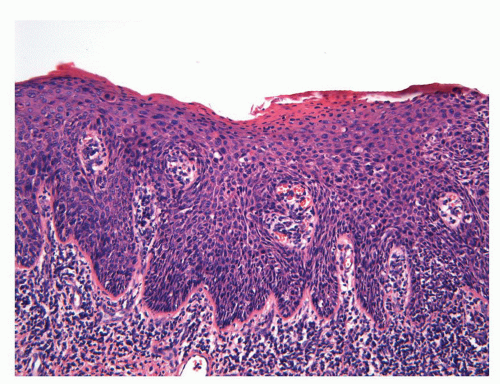 FIGURE 14-31 ▪ Undifferentiated PeIN, basaloid type. There is replacement of the epithelium by a monotonous proliferation of small round cells with high nuclear/cytoplasmic ratio. |
Some authors still use the old terminology of mild (PeIN 1), moderate (PeIN 2), and severe dysplasia (PeIN 3 or carcinoma in situ) based on the degree, in thirds, that the epithelium is atypical.37,38,113, 114, 115, 116 Because of the overlapping features between moderate and severe dysplasia, PeIN 2 and 3 are combined in one category in some systems. Such systems divide PeIN into only two grades: low and high. These systems mainly apply to the HPV-related group of lesions.
It has been suggested that the use of a triple p16/p53/Ki-67 immunohistochemical panel may be helpful in the classification, differential diagnosis, and morphologic standardization of penile intraepithelial lesions.112
Bowenoid Papulosis
Bowenoid papulosis is a multifocal HPV-related condition affecting the anogenital region of young adults.126, 127, 128, 129 Clinically, penile bowenoid papulosis is characterized by multiple soft papules or macules mostly affecting the skin of the shaft, and less frequently the epithelium of the glans, sulcus, or foreskin. Despite the clinical benign-looking appearance of these papular lesions, histopathologic findings reveal features of a squamous cell carcinoma in situ.37,38 There is a proliferation of atypical cells with a high nuclear/cytoplasmic ratio that tend to have a more patchy and less continuous disposition when compared with carcinoma in situ (Fig. 14-35). There may be a variable increased pigmentation of the basal layer.127 Definitive histologic distinction between carcinoma in situ and bowenoid papulosis is not possible; therefore, clinical correlation is necessary to confirm this diagnosis. Immunosuppression, including HIV infection, greatly increases the risk for bowenoid papulosis, with the lesions in this setting tending to be more widespread. Lesions of bowenoid papulosis may regress spontaneously or (especially in immunocompromised patients) may evolve to invasive carcinoma.128 High-risk HPVs, mainly HPV-16, are regularly found in the lesions.129
Squamous Cell Carcinoma
The majority of penile carcinomas are squamous cell carcinomas that arise on the mucosal squamous epithelium of the distal portion of the organ including glans, coronal sulcus, and foreskin (Fig. 14-36).3




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree