Chapter 44 Pancreaticobiliary Pain and Suspected Sphincter of Oddi Dysfunction
Video for this chapter can be found online at www.expertconsult.com.
Definitions
Attempts have been made to develop consensus on defining the signs and symptoms of SOD culminating in what are called the “Rome criteria.”1 Definitions established for postcholecystectomy patients and those with gallbladder in situ are listed in Box 44.1. Revisions in the Rome criteria for SOD were recently published.2 The Rome criteria are meant to provide a general framework for clinicians but obviously do not describe all patients. A unifying symptom, present in all patients with SOD, is pain. There may be associated symptoms such as nausea with or without vomiting but the hallmark symptom is pain—located in the epigastrium and/or right upper quadrant (RUQ). When evaluating a patient with possible SOD, the most important aspect of the evaluation is the history. It is imperative that the clinician gain a clear understanding of the nature, location, and timing of pain. The Rome criteria specify that the pain should be intermittent with pain-free intervals. This is a very controversial point. While biliary pain is typically intermittent, in some cases patients will have a constant, low-grade discomfort with exacerbations. This can be seen particularly in those with pancreatic sphincter hypertension who typically have exacerbations after eating. These patients should undergo careful review and extensive evaluation for other causes of pain (Box 44.2) but should not be excluded from evaluation for SOD based solely on there being a constant component to their pain. However, if associated symptoms such as nausea, vomiting, abdominal distension, or bowel dysfunction are dominant, the patient likely does not have SOD as the predominant explanation for his or her symptoms.
Box 44.1
Rome Criteria for Sphincter of Oddi Dysfunction
Postcholecystectomy
 Episodes lasting 30 minutes or longer.
Episodes lasting 30 minutes or longer.
 Recurrent symptoms occurring at different intervals and not daily.
Recurrent symptoms occurring at different intervals and not daily.
 The pain is steady, interrupts daily activity, and/or leads to medical encounter.
The pain is steady, interrupts daily activity, and/or leads to medical encounter.
 The pain is not relieved by bowel movements, postural change, or antacids.
The pain is not relieved by bowel movements, postural change, or antacids.
 Structural diseases that could explain symptoms are excluded.
Structural diseases that could explain symptoms are excluded.
Based on observations and after developing correlations between patients’ presentation and outcomes after endoscopic sphincterotomy, Joseph Geenen, Walter Hogan, and Wylie Dodds published what have come to be known as the “Geenen-Hogan criteria” (Table 44.1).3 These have been modified over the years but still serve as a very good compass to clinicians to direct them in their evaluation and therapeutic decision making. The original criteria were applied to patients who had previously undergone cholecystectomy and were based on three factors that could be assessed without endoscopic retrograde cholangiopancreatography (ERCP): presence of “typical” pancreatic- or biliary-type pain, the presence or absence of elevated liver or pancreatic tests during or shortly following an episode of pain, and the presence or absence of bile and/or pancreatic duct dilation. The original criteria also included measurement of pancreatic and biliary drainage times. Drainage times are very imprecise and require instillation of contrast into the respective duct, and in the case of biliary drainage times the endoscope must be withdrawn, the patient placed in the supine position, and an abdominal film obtained at 45 minutes. Studies have shown that drainage times do not correlate with SOM4 and that delayed drainage is common in asymptomatic postcholecystectomy volunteers.5 As a result, drainage times are no longer performed and are not part of the current Geenen-Hogan (G-H) criteria.
Clinical Evaluation
The first step is a detailed review of prior health care encounters pertinent to the clinical presentation with a focus on questions of when, where, and what (Box 44.3). A complete history and thorough review of records will define the clinical symptoms, reveal what tests have been done, what treatments (surgical, endoscopic, medical) have been tried, and what the impact has been on the patient. Patients with unexplained symptoms that may be attributed to SOD often end up undergoing a massive assault on both diagnostic and therapeutic fronts. It can be helpful to organize objective data regarding prior laboratory testing, imaging, and treatments (Box 44.4).
The possibility of more common and potentially more treatable diagnoses should be considered before proceeding with an evaluation for possible SOD. Symptom history and diagnostic testing should be directed at evaluation for the potential diagnoses listed in Box 44.2. For example, bile duct dilation should raise a suspicion for neoplasia or bile duct stones if associated with persistently abnormal liver tests. Alternatively, a dilated bile duct with normal liver tests in a patient with intermittent pain should raise suspicion for SOD. Evaluation for possible common bile duct stones deserves careful consideration. Bile duct stones are very rarely found when routine imaging tests such as transabdominal ultrasound and laboratory testing are normal. Therefore unless there are objective indicators to suggest bile duct pathology, ERCP should be avoided when purely used to “rule out bile duct stones.” Additional imaging such as magnetic resonance cholangiopancreatography (MRCP) or endoscopic ultrasound (EUS) can be helpful in this setting. It is most reasonable to consider ERCP when SOM and/or definitive endoscopic therapy is planned. In this era of the obesity epidemic, one must also be careful to evaluate patients with abdominal pain and “abnormal” liver tests. Persistently elevated liver tests in an obese patient with upper abdominal pain are more likely to be related to fatty liver or bile duct stones than to SOD.
Ideally, patients with unexplained upper abdominal pain can be categorized as to the likelihood for SOD and a favorable response to endoscopic treatment. The G-H classification (Table 44.1) is the standard in this regard. Type I SOD patients have objective evidence of impaired drainage and are more likely to have structural obstruction (papillary stenosis). In addition to characteristic pain, they have dilated ducts and abnormal liver tests during episodes of pain.
Patients with Type II SOD have characteristic pain and either a dilated duct or abnormal laboratory tests with pain. Type III SOD patients have typical biliary or pancreatic pain but no objective evidence of impaired drainage. Such patients likely have a purely functional disorder. This categorization of patients is important because it predicts, to a certain extent, the chance of finding an abnormal SOM and having a favorable outcome following sphincterotomy (Table 44.2).6
Table 44.2 Correlation between Geenen-Hogan Criteria, Results of SOM, and Outcome with Sphincterotomy

Once a clinical impression of SOD is established, ideally a noninvasive test can confirm one’s clinical impression before proceeding to ERCP. Several tests have been studied and individual centers have reported good correlation with SOM and/or sphincterotomy. The problem is that when these tests are evaluated on a broader scale, their accuracy does not match previous, single-center reports. The Hopkins group first reported on the accuracy of dynamic (quantitative) biliary scintigraphy.7,8 The test was designed to measure delayed bile flow through the ampulla by assessing the time it takes for the radionuclide to reach the duodenum. These authors found a good correlation with SOM. Their results were supported by Corazziari et al.9 This prompted the Hopkins group to suggest that this test could substitute for SOM.10 However, when this test was evaluated in normal volunteers, we found that it had very poor specificity and little value in excluding SOD in patients suspected to suffer from this disorder.11
Another test hypothesized to detect SOD is fatty meal sonography (FMS). An abnormal test is defined by >2 mm dilation of the bile duct 45 minutes after ingestion of a standardized “fatty meal.” Rosenblatt et al. compared SOM, FMS, and hepatobiliary scintigraphy (HBS) in a retrospective comparative study.12 Poor correlation was observed between FMS and HBS with SOM. However, among the patients with abnormal SOM who had a good long-term response to sphincterotomy, 85% (11 of 13) had an abnormal FMS and HBS. This raises an interesting point: Perhaps noninvasive tests should be evaluated as to whether they predict response to sphincterotomy rather than as to whether they correlate with SOM. What a clinician really wants to know from a noninvasive test is whether or not the patient will respond to endoscopic sphincterotomy.
Upper Abdominal Pain with Gallbladder In Situ
Management of patients with biliary-type pain without evidence of gallstones on standard imaging represents a challenge. Physicians (including surgeons) and patients usually prefer to identify some proof of gallbladder pathology before considering cholecystectomy. Biliary crystal analysis can be performed on bile collected from the duodenum or bile duct after cholecystokinin (CCK) stimulation. Endoscopic ultrasound is more sensitive for discovering biliary sludge13,14 and can also be used to assess for evidence of pancreatitis. If EUS and CCK-stimulated biliary drainage are performed and biliary crystals or gallbladder sludge is found, more than 90% of patients will have resolution of pain with cholecystectomy.15 Biliary scintigraphy may reveal evidence of chronic acalculous cholecystitis (gallbladder ejection fraction <35%).16 Empiric cholecystectomy, however, will benefit about three fourths of those patients with classic biliary pain, independent of other testing.17–21
The exact role for SOM in this setting is not established. There has been limited study of the prevalence of SOD in patients with gallbladder in situ. Guelrud reported on 121 patients with biliary pain and a finding of gallstones but a normal caliber bile duct by ultrasound.22 ERCP and SOM were performed and he found elevated basal sphincter pressures in 14 patients (11.6%). Interestingly, 4% of patients in this group with a normal alkaline phosphatase had elevated basal sphincter pressures while 40% with an elevated alkaline phosphatase were found to have SOD. Ruffolo et al. investigated 81 patients with typical biliary-type pain and a normal gallbladder ultrasound.23 When ERCP and SOM were performed, 53% of these patients had SOD as diagnosed by elevated basal sphincter pressures. For the whole group, 49% had an abnormal ejection fraction on gallbladder scintigraphy but the finding of SOD did not correlate with ejection fraction. All patients in this group with elevated sphincter pressures underwent biliary sphincterotomy and the short-term results of pain relief (1 year) were quite good. However, with longer-term follow-up most patients ultimately required cholecystectomy.24
Sphincter of Oddi Manometry
Equipment
1. All of the “normal” data have been generated with a water-perfused system.
2. The electronic manometry catheters are expensive and fragile.
Advances have been made in water-perfused systems, particularly in the software, which makes setup, recording, and interpreting the manometry much easier. These systems are available through Sandhill Scientific (Highlands Ranch, Colo.) and Medtronic (Minneapolis, Minn.), as well as other manufacturers. The entire system, consisting of the computer and the water perfusion system, can be placed on a small cart and is readily mobile.
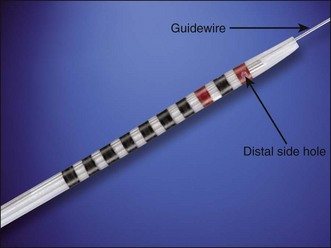
The original catheter used for SOM was manufactured by Arndorfer (Greendale, Wis.) and some practitioners still use these catheters. However, the majority of catheters used in SOM are manufactured by Cook Endoscopy (Winston-Salem, N.C.). The catheter consists of three lumens, two of which terminate in a side hole of the catheter while the third lumen has both a side port and an end port (Fig. 44.1). The lumen with the end port does accommodate a 0.018- or 0.021-in guidewire. All three channels can be used for the manometry recording but a randomized study showed that sacrificing the third lumen with the side and end ports, and using that for aspiration during a pancreatic manometry, significantly reduced the postmanometry pancreatitis rate.25 It was found that aspiration during manometry of the biliary sphincter was not necessary.26 Water is perfused at 0.25 mL per minute through each port. The triple-lumen manometry catheter made by Cook Endoscopy consists of Teflon and is tapered at the end. In the distal end of the catheter there are black rings spaced 1 mm apart. Moving proximally to distally there are seven black rings followed by a red ring, a black ring, and another red ring in sequence. The rings allow communication between the endoscopists and the manometry assistant to record the position of the catheter relative to the papilla orifice. The proximal end of the catheter (that portion that is outside the scope channel) is bolstered by an additional plastic coating that helps stiffen the catheter and prevents kinking as the catheter is inserted and withdrawn. The catheter is supplied in two types: the so-called “short-nose” and “long-nose.” The short-nose catheter has 5 mm between the last black ring and the tip of the catheter. The length of the distal tip on the long-nose catheter is 20 mm. The main advantage of the long-nose catheter is that the manometry can be completed (withdrawn to the last ring) while maintaining the cannulation. The downside of this catheter, in the opinion of these authors, is that the long-nose catheter is harder to cannulate with.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree