Chapter 51 Pancreatic Interventions in Acute Pancreatitis
Ascites, Fistulae, Leaks, and Other Disruptions
Background
The role of endoscopic retrograde cholangiopancreatography (ERCP) in the setting of acute pancreatitis has primarily been twofold.1 First, it has been used after resolution of an acute attack, or more commonly multiple attacks, in an attempt to define etiology. Congenital variants, including duodenal duplication, anomalous pancreaticobiliary union, annular pancreas, and pancreas divisum, can be diagnosed, as can other anatomic causes of pancreatitis such as ampullary adenoma or surreptitious stone disease. For the most part, less invasive approaches to imaging the pancreatic duct (PD) such as endoscopic ultrasound (EUS), magnetic resonance imaging (MRI), and magnetic resonance cholangiopancreatography (MRCP) have supplanted the need for ERCP, a procedure that can actually cause the disease for which it is being applied.2 In patients with “idiopathic” relapsing acute pancreatitis, most series suggest that sphincter of Oddi dysfunction (SOD) is the most common etiology when other diagnostic studies have been exhausted. As such, there remains a significant role for ERCP in conjunction with sphincter of Oddi manometry (SOM) in such patients (Box 51.1).
Box 51.1 Key Points
Introduction
 With the exception of sphincter of Oddi dysfunction, the use of ERCP to diagnose the etiology of relapsing attacks of pancreatitis has been supplanted by pancreas protocol CT, MRI/MRCP, and EUS.
With the exception of sphincter of Oddi dysfunction, the use of ERCP to diagnose the etiology of relapsing attacks of pancreatitis has been supplanted by pancreas protocol CT, MRI/MRCP, and EUS.
 ERCP in the setting of acute pancreatitis is most commonly performed for biliary tract intervention.
ERCP in the setting of acute pancreatitis is most commonly performed for biliary tract intervention.
 Biliary intervention in pancreatitis may be performed not only to treat biliary calculi but also for palliation of biliary obstruction from pancreatic edema and fluid collections.
Biliary intervention in pancreatitis may be performed not only to treat biliary calculi but also for palliation of biliary obstruction from pancreatic edema and fluid collections.
 Therapeutic pancreatography in acute pancreatitis includes bypass of ductal obstructions and treatment of leaks and their consequences and should be undertaken as one aspect of a multidisciplinary approach.
Therapeutic pancreatography in acute pancreatitis includes bypass of ductal obstructions and treatment of leaks and their consequences and should be undertaken as one aspect of a multidisciplinary approach.
The second role that ERCP has played is in the treatment of acute biliary pancreatitis.3,4 This subject is covered in detail in Chapter 50. However, selective use of ERCP in patients with presumed biliary pancreatitis who have high suspicion for choledocholithiasis or biliary sepsis seems to be common clinical practice.
Video for this chapter can be found online at www.expertconsult.com.
In addition to its application in conjunction with SOM in patients with acute relapsing pancreatitis (ARP) and its selective application in biliary pancreatitis, ERCP has been used as a means to provide pancreatic endotherapy in the setting of severe or smoldering pancreatitis related to ductal disruption or of spasm or edema of the sphincter of Oddi.5–7 This chapter focuses on these latter indications, although it should be noted that ductal disruptions are seen in a background of chronic as well as acute pancreatitis. Moreover, in contrast to controlled observations about the timing and appropriateness of ERCP in acute relapsing or severe biliary pancreatitis, respectively, most publications related to pancreatic endotherapy during an attack of pancreatitis have been uncontrolled and anecdotal but also often dramatic.
Epidemiology of Ductal Disruption
If the underlying pathophysiology of acute pancreatitis is colocalization of zymogen granules with cell membranes, setting off an inflammatory cascade with local effects related to cytokine release and recruitment of inflammatory cells, it seems reasonable that this sequence antedates disruption of ductular epithelial cells and subsequent pancreatic juice leak in most cases of acute pancreatitis.5,8 However, acute sphincter obstruction in the setting of a common bile duct stone may increase intraductal pressure leading to side branch or acinar leak with resultant pancreatitis. Likewise, any other downstream obstruction may increase upstream duct pressure leading to PD blowout and perpetuation or exacerbation of pancreatitis.9,10 In acute pancreatitis this is most commonly seen with severe edema whereas in chronic pancreatitis disruptions are usually the consequence of a downstream stricture or stone and resultant ductal hypertension. In the setting of severe pancreatic necrosis, ductal disruption is almost invariable, although whether the ductal disruption is the cause or the consequence of the necrosis remains ill defined.10,11 The presence of a peripancreatic fluid collection does not imply a significant ongoing leak in all instances. Whereas up to 40% of patients with acute pancreatitis develop a fluid collection, less than 5% of these patients go on to develop a pseudocyst.12
Anatomic Classification
Although this chapter focuses on pancreatic duct leaks and their endoscopic treatment, Box 51.2 summarizes some of the other endoscopically amenable lesions that endoscopists see in a busy ERCP practice. They include bile duct obstruction from stones, edema within the head of the pancreas, and neoplasms that occasionally present with pancreatitis. From a pancreatic standpoint, they include neoplastic obstruction of the papilla or duct, edema or spasm of the sphincter mechanism, and an inflammatory PD stenosis.
Box 51.2
Endoscopically Amenable Anatomic Lesions Seen in Acute Pancreatitis
 Biliary obstruction: jaundice, cholangitis
Biliary obstruction: jaundice, cholangitis
 Pancreatic duct leak: exacerbation/perpetuation of pancreatitis
Pancreatic duct leak: exacerbation/perpetuation of pancreatitis
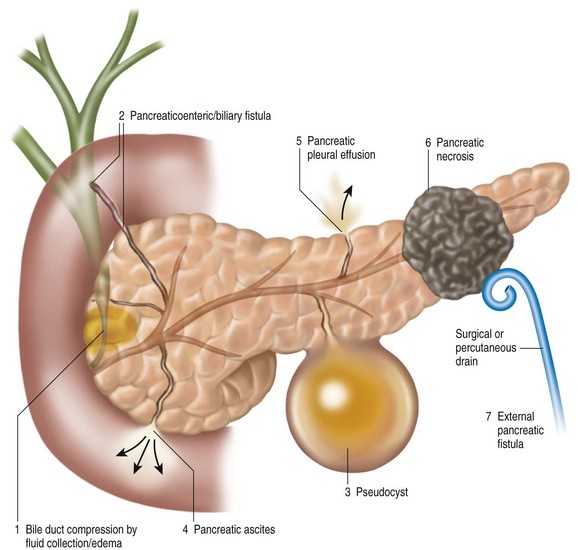
Pancreatic duct leaks can be defined anatomically by site of disruption, which in conjunction with the size of the leak and the presence or absence of concomitant necrosis often determines the clinical manifestations (Fig. 51.1).8,12,13 As such, a major blowout of the PD tail may cause an acute perisplenic fluid collection with or without a high-amylase, left pleural effusion. Alternatively, pancreatic juice may follow anatomic pathways around the left kidney and even into the pelvis, with resultant scrotal or labial edema. Blowouts in the head of the pancreas may be associated with C-loop edema and gastric outlet obstruction, biliary compression, or even pancreaticobiliary fistulization, right perinephric fluid accumulation, and dissection into the pelvis or perihilar area. A central disruption may result in fluid collection within the lesser sac, dissection into the mediastinum or pericardium, and when associated with significant central pancreatic necrosis, a permanently disconnected duct/gland syndrome.13
Anatomic classifications based on the presence of an acute or chronic pancreatic duct leak are outlined in Box 51.3. Classically, pancreatic duct leaks (fistulae) are classified as internal or external, the latter almost always a consequence of trauma, surgery, or interventional radiologic drainage procedures.14–18 Internal fistulae, in turn, classically have included pseudocysts, pancreatic ascites, high-amylase pleural effusions, and erosion of pancreatic fluid collections into contiguous organs, resulting in pancreaticoenteric, gastric, colic, or biliary fistulae.19–23 They also include evolving pancreatic necrosis in which variable amounts of high-amylase fluid collect, usually in the context of central pancreatic necrosis.
Management Strategies (Box 51.4)
Diagnosis
The management of pancreatic fistulae presupposes their diagnosis. The diagnosis of external fistulae is usually self-evident and consists of variable output of clear pancreatic juice following percutaneous drainage of a pseudocyst or peripancreatic fluid collection (Box 51.5).1 Alternatively, persistent output from a Jackson-Pratt (JP) drain following pancreatic resection, decompression, or peripancreatic surgery (e.g., splenectomy, left nephrectomy, right hemicolectomy, or gastrectomy) is not usually a subtle manifestation of an external leak. More troublesome, however, may be the patient who sustains a penetrating abdominal injury such as a knife or gunshot wound in whom the external fistula is overlooked because of concerns of more significant injury.
Box 51.4 Key Points
Management Strategies
 The diagnosis of external pancreatic fistulae is usually self-evident.
The diagnosis of external pancreatic fistulae is usually self-evident.
 Pancreas protocol CT scan is most often the best way to define the consequences (fluid collection, necrosis) of an internal pancreatic fistula.
Pancreas protocol CT scan is most often the best way to define the consequences (fluid collection, necrosis) of an internal pancreatic fistula.
 Unless endotherapy can be done at the time of an ERCP, secretin-enhanced MRCP may be a better test to define the location or persistence of an internal pancreatic fistula.
Unless endotherapy can be done at the time of an ERCP, secretin-enhanced MRCP may be a better test to define the location or persistence of an internal pancreatic fistula.
Box 51.5
Diagnostic Studies in Pancreatic Duct Leaks
The diagnosis of internal fistulae is outlined in Box 51.5. In essence, noninvasive imaging, particularly pancreas protocol computed tomography (CT), remains the best initial diagnostic test in patients with smoldering or severe pancreatitis or in patients with underlying chronic pancreatitis and an acute exacerbation of symptoms.13 Not only will CT define the consequences of pancreatitis (fluid collections, necrosis, effusions, ascites)24 but it can also be used to define the potential etiology (e.g., stones or strictures) as well as follow the subsequent evolution of pancreatitis. CT remains an imperfect tool, however, in that biliary stone disease is underestimated, the fluid component associated with evolving pancreatic necrosis is overestimated, and leaks are implied rather than defined.25 Further confirmation of a ductal disruption may require sequential scans demonstrating an enlarging fluid collection, aspiration of that fluid collection with measurement of amylase or lipase, an ERCP demonstrating presence and location of the leak, or secretin-enhanced MRCP (S-MRCP). The latter study has been shown to be predictive of ongoing ductal disruption and clearly minimizes potential ERCP adverse events such as exacerbation of pancreatitis and iatrogenic infection of an undrained fluid collection.26 It may also demonstrate patients with a complete ductal disruption and a disconnected gland syndrome in whom leak closure by ERCP alone is unlikely to be successful. Finally, use of S-MRCP prior to ERCP may help to define subsequent endoscopic management comparable to its use in hilar neoplasms of the liver. ERCP, in turn, is usually definitive in showing not only the site of the ductal disruption (if persistent) but also the proximate cause or reason for persistence (PD stones, inflammatory or fibrotic structure).13,27 For the most part, however, diagnostic pancreatography adds an unnecessary risk to the care of acutely or chronically ill patients with presumptive leak, unless endoscopic, percutaneous, or surgical therapy is contemplated.28
Management (Box 51.6)
The presence of a presumptive pancreatic fistula is not a demand to undertake endotherapy. Important considerations include whether the patient has acute or underlying chronic pancreatitis, whether pancreatic necrosis is present or absent, whether there is superinfection of a fluid collection, whether the presumptive leak is accessible to endoscopic control, and whether the leak is controlled at the time of presentation. For instance, the vast majority of low-volume leaks following pancreatic resection are low grade, are controlled by a surgically placed JP drain, and spontaneously close with or without concomitant octreotide over days or several weeks.29,30 On the other hand, a patient may have rapidly increasing ascites or pleural effusion or concomitant jaundice or cholestasis that demands urgent attention.
Box 51.6 Key Points
Management of Pancreatic Fistulae
 The appropriate management of pancreatic fistulae should include a multidisciplinary team.
The appropriate management of pancreatic fistulae should include a multidisciplinary team.
 Transpapillary stent placement has a markedly higher success rate in treating internal fistulae if the disruption is bridged.
Transpapillary stent placement has a markedly higher success rate in treating internal fistulae if the disruption is bridged.
 The role of ERCP in patients with an obvious disconnected gland syndrome is limited.
The role of ERCP in patients with an obvious disconnected gland syndrome is limited.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree