Renal cell tumors
Papillary adenoma
Oncocytoma
Clear cell renal cell carcinoma
Multilocular cystic clear cell renal cell neoplasm of low malignant potentiala
Papillary renal cell carcinoma
Chromophobe renal cell carcinoma
Hybrid oncocytic/chromophobe tumora
Carcinoma of the collecting ducts of Bellini
Renal medullary carcinoma
MiT family translocation renal cell carcinomaa
Xp11 translocation renal cell carcinoma
t(6;11) renal cell carcinoma
Carcinoma associated with neuroblastoma
Mucinous tubular and spindle cell carcinoma
Tubulocystic renal cell carcinomaa
Acquired cystic disease-associated renal cell carcinomaa
Clear cell (tubulo) papillary renal cell carcinomaa
Hereditary leiomyomatosis renal cell carcinoma syndrome-associated renal cell carcinomaa
Renal cell carcinoma, unclassified
Metanephric tumors
Metanephric adenoma
Metanephric adenofibroma
Metanephric stromal tumor
Nephroblastic tumors
Nephrogenic rests
Nephroblastoma
Cystic partially differentiated nephroblastoma
Mesenchymal tumors
Occurring mainly in children
Clear cell sarcoma
Rhabdoid tumor
Congenital mesoblastic nephroma
Ossifying renal tumor of infants
Occurring mainly in adults
Leiomyosarcoma (including renal vein)
Angiosarcoma
Rhabdomyosarcoma
Malignant fibrous histiocytoma
Hemangiopericytoma
Osteosarcoma
Synovial sarcomaa
Angiomyolipoma
Epithelioid angiomyolipomaa
Leiomyoma
Hemangioma
Juxtaglomerular cell tumor
Renomedullary interstitial cell tumor
Schwannoma
Solitary fibrous tumor
Mixed mesenchymal and epithelial tumors
Cystic nephroma/mixed epithelial stromal tumor
Neuroendocrine tumors
Carcinoid (low-grade neuroendocrine tumor)
Neuroendocrine carcinoma (high-grade neuroendocrine tumor)
Primitive neuroectodermal tumor
Neuroblastoma
Pheochromocytoma
Hematopoietic and lymphoid tumors
Lymphoma
Leukemia
Plasmacytoma
Germ cell tumors
Teratoma
Choriocarcinoma
Metastatic tumors
Other tumors
Several new subtypes of RCC are recognized in the ISUP Vancouver classification such as tubulocystic RCC, acquired cystic disease-associated RCC, clear cell (tubulo) papillary RCC, MiT family translocation RCC (including t(6;11) RCC), and hereditary leiomyomatosis RCC syndrome-associated RCC. Some newly described RCCs are also considered as provisional entities such as thyroid-like follicular RCC, succinic dehydrogenase B deficiency-associated RCC, and ALK-translocation RCC. Additional data are needed to help shed light on the biology of these rare unique tumors. Some innovations were also made on traditional tumor entities, such as renaming multicystic clear cell RCC as a neoplasm of low malignant potential, subtyping papillary RCC into type 1 or 2, accepting the hybrid oncocytic/chromophobe tumor as a discrete subtype of chromophobe RCC, and merging cystic nephroma with mixed epithelial stromal tumor into one tumor spectrum. Despite the inclusion of these novel entities, clear cell RCC, papillary RCC, and chromophobe RCC still comprise >90 % of the RCCs. The proportion of MiT family translocation RCC however is higher in pediatric and young adult patients.
Staging of Renal Cancers
Introduction
The tumor node metastasis (TNM) is the most widely accepted staging system for renal cancer [2]. This approach measures the extent of cancer spread at the primary organ site, regional lymph nodes, and distant sites (Table 4.2). The TNM system underwent considerable revisions over the past three decades with its latest edition (seventh) published in 2010, and a new version is being expected within the next 2 years as of 2015. Purely based on the tumor’s anatomic extent, the different TNM stage categories are lumped into four main prognostic groups (Table 4.3). The clinical (c) stage is routinely used as a guide in determining the type of primary management , such as nephron sparing surgery (NSS) or ablative therapies for low stage renal tumors or systemic therapy for advanced stage tumors. The pathologic (p) stage mainly provides prognosis of outcome after surgical resection of renal cancer and is important on the decision for adjuvant therapy. TNM stage is often incorporated in the inclusion criteria and in stratifying patients for clinical therapeutic trials. The accuracy of TNM stage in renal cancer can be further enhanced by its integration in the different prognostic and predictive models such as the MSKCC prognostic nomogram; the Mayo Clinic stage, size, grade, and necrosis (SSIGN) score; and the UCLA integrated staging system (UISS) [3–7].
Table 4.2
Definitions of the 2010 AJCC TNM staging for renal cancers
Primary tumor (T) | |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
T1 | Tumor 7 cm or less in greatest dimension, limited to the kidney |
T1a | Tumor 4 cm or less in greatest dimension, limited to the kidney |
T1b | Tumor more than 4 cm but not more than 7 cm in greatest dimension, limited to the kidney |
T2 | Tumor more than 7 cm in greatest dimension, limited to the kidney |
T2a | Tumor more than 7 cm but less than or equal to 10 cm in greatest dimension, limited to the kidney |
T2b | Tumor more than 10 cm, limited to the kidney |
T3 | Tumor extends into major veins or perinephric tissues but not into the ipsilateral adrenal gland and not beyond Gerota’s fascia |
T3a | Tumor grossly extends into the renal vein or its segmental (muscle-containing) branches, or tumor invades perirenal and/or renal sinus fat but not beyond Gerota’s fascia |
T3b | Tumor grossly extends into the vena cava below the diaphragm |
T3c | Tumor grossly extends into the vena cava above the diaphragm or invades the wall of the vena cava |
T4 | Tumor invades beyond Gerota’s fascia (including contiguous extension into the ipsilateral adrenal gland) |
Regional lymph nodes (N) | |
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Metastasis in regional lymph node (s) |
Distant metastasis (M) | |
M0 | No distant metastasis |
M1 | Distant metastasis |
Table 4.3
2010 AJCC TNM anatomic stage or prognostic groupings
Stage I | T1 | N0 | M0 |
Stage II | T2 | N0 | M0 |
Stage III | T1 or T2 | N1 | M0 |
T3 | N0 or N1 | M0 | |
Stage IV | T4 | Any N | M0 |
Any T | Any N | M1 |
Historical Background
In 1958, Flocks and Kasdesky [8] introduced one of the first formal stagings for renal cancer based on the tumor’s anatomic extent and patterns of spread. A year later, Petkovic [9] proposed a similar classification that subdivided intrarenal tumors into stages I and II (Flocks and Kasdesky’s stage I). In the 1960s, Robson modified these systems, incorporated venous involvement, and subdivided localized extrarenal spread [10, 11]. However, Robson’s system was hampered by inaccuracies in some of the stage definitions due to the lumping of prognostically different patterns of anatomic spread [11].
First developed in the 1940s by Pierre Denoix in France, the TNM system was adopted by the Union Internationale Contre le Cancer (UICC) , while the American Joint Commission on Cancer (AJCC) used a slightly different classification. In 1987, the UICC and AJCC were unified, and the first major revision of the TNM staging was published that incorporated tumor size cutoffs derived from cross-sectional imaging studies. Since then, the TNM system underwent several major revisions published in 1993 (supplement), 1997, 2002, and the latest in 2010, building on experiences and evidences accumulated from each prior version in order to enhance its prognostic accuracies (Table 4.4). Revisions in the 2010 TNM system include T2 tumors divided into T2a (>7 cm but ≤10 cm) and T2b (>10 cm); ipsilateral adrenal gland contiguous invasion classified as T4 and, if not contiguous as M1, renal vein involvement reclassified as T3a; and nodal involvement simplified into N0 and N1 [2].
Table 4.4
Evolution of renal cancer staging system
Stage | Robson (1969)a | UICC/AJCC TNM, fourth edition (1987) | UICC/AJCC TNM, fifth edition (1997) | UICC/AJCC TNM, sixth edition (2002) | UICC/AJCC TNM, seventh edition (2010) |
|---|---|---|---|---|---|
T1 | (I) Organ confined, any size | Organ confined, ≤2.5 cm | Organ confined, ≤7 cm | – | – |
T1a | – | – | – | Organ confined, ≤4 cm | Organ confined, ≤4 cm |
T1b | – | – | – | Organ confined, >4–7 cm | Organ confined, >4–7 cm |
T2 | (II) Into perinephric tissue | Organ confined, >2.5 cm | Organ confined, >7 cm | Organ confined, >7 cm | – |
T2a | – | – | – | – | Organ confined, >7–10 cm |
T2b | – | – | – | – | Organ confined, >10 cm |
T3a | (IIIa) Renal vein | Perinephric tissue or contiguous adrenal gland extension | Perinephric tissue or contiguous adrenal gland extension | Perinephric or sinus tissue or contiguous adrenal gland extension | Perinephric or sinus tissue or renal vein or its segmental branches |
T3b | (IIIb) Node involvement | Renal vein | Renal vein or vena cava below diaphragm | Renal vein or vena cava below diaphragm | Vena cava below diaphragm |
T3c | (IIIc) Both renal vein and node involvement | Vena cava below diaphragm | Vena cava above diaphragm | Vena cava above diaphragm | Vena cava above diaphragm or wall of vena cava at any level |
T4 | – | – | Beyond Gerota’s fascia | Beyond Gerota’s fascia | Beyond Gerota’s fascia or contiguous adrenal gland extension |
T4a | (IVa) Invasion of adjacent structures | Beyond Gerota’s fascia | – | – | – |
T4b | (IVb) Distant metastasis | Vena cava above diaphragm | – | – | – |
Components of the TNM Staging System for Renal Cancer
Organ-Confined Tumors
Tumors 7 cm or Smaller (T1)
Since the 2002 TNM version, T1 tumors are subdivided into T1a and T1b using 4 cm size cutoff (Figs. 4.1 and 4.2). This was mainly based on the study by Hafez et al. [12] wherein they reviewed 485 patients with localized renal cancer treated with NSS and showed a more favorable cancer-free survival in tumors ≤4 cm compared to larger tumors. Since then, the prognostic impact of this subdivision has been validated in subsequent studies [13–17]. Despite of several studies suggesting a different optimal size cutoff for T1a and T1b and T1 versus T2, the 4 and 7 cm cutoffs are retained in the current 2010 TNM system [18–22]. By incidence, most renal cancers are diagnosed as T1 tumors (~55 to 70 %) and with greater T1a (35–45 %) than T1b cases (19–27 %) (Table 4.5) [23–25]. A practical usefulness of this T1 grouping is that most of the current guidelines recommend NSS for T1 renal cancer when technically feasible, and this recommendation is generally accepted for T1a tumors.
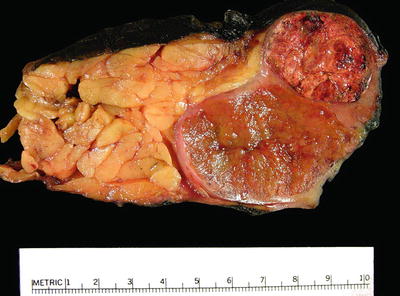
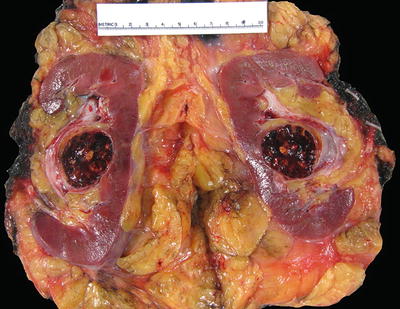

Fig. 4.1
Kidney with two synchronous organ-confined tumors . The smaller tumor (top) is T1a and the larger tumor (bottom) is T1b. Multiple tumors are staged according to the highest T stage, in this case as T1b

Fig. 4.2
T1a clear cell RCC that is very close to the hilum. In this case, adequate sampling of the tumor-sinus interface is important to ascertain the absence of invasion (T3a)
Table 4.5
Distribution of renal cancer patients by pathologic T stage
pTstage | Novara et al. (2010) [23] | Lee et al. (2011) [24] | Pichler et al. (2013) [25] |
|---|---|---|---|
N | 5339 | 1691 | 2739 |
T1a (%) | 35.5 | 45.3 | 35.7 |
T1b (%) | 27
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





