Fig. 8.1
Cancer-specific survival among patients diagnosed with CDC or RMC as reported by Abern et al. The x-axis represents the proportion of patients surviving and the y-axis represents time in months. Patients with RMC had nearly three times the hazard of dying compared to CDC patients [3]. CSS cancer-specific survival (permission granted by Elsevier. Abern et al. [3])
When patient and tumor characteristics between CDC and RMC are compared, RMC patients are significantly younger, more often African American, and more likely to have lymph node involvement and distant metastatic disease at presentation, and median ca ncer-specific survival is significantly shorter [3]. Distinguishing clinical features in CDC and RMC are summarized in Table 8.1.
Table 8.1
Distinguishing clinical features in collecting duct carcinoma versus renal medullary carcinoma
Feature | Collecting duct carcinoma | Renal medullary carcinoma |
|---|---|---|
Average age | 63 years | 19 years |
Race | ||
Caucasian | 71 % | 5 % |
African American | 23 % | 90 % |
Association with sickle cell hemoglobinopathy | No | Yes |
Metastatic at presentation | 28 % | 71 % |
Median survival | 30 months | 5 months |
Radiographic Features
The usual CT findings of C DC are of a solid renal mass located in the medulla with involvement of the renal sinus, infiltrative growth, preserved renal contour, and a cystic component [10]. Weak and heterogeneous enhancement due to areas of necrosis, hemorrhage, and calcification can be seen [11]. RMC is also characterized by an infiltrative, medullary-based solid renal mass with heterogeneity due to areas of hemorrhage and necrosis, and caliectasis is often present [11, 12]. Regional lymphadenopathy and metastasis are frequently seen in both CDC and RMC at initial diagnosis. These findings are nonspecific and do not allow for differenti ation from more common types of RCC by imaging.
Pathologic Characteristics of Collecting Duct and Renal Medullary Carcinoma
Collecting Duct Carcinoma
Gross Pathology
On gross examination, CDCs are white to grey, firm, multinodular tumors with infiltrative borders and focal areas of tumor necrosis (Fig. 8.2), but without significant areas of hemorrhage, making them grossly distinct from the more usual types of RCCs. When small, the tumors appear centered in the renal medulla; however, this may be difficult to appreciate in larger tumors, which can extend into the renal cortex, renal pelvis, perinephric fat, or renal hilum [7, 13]. Tumors average about 6 cm in diameter and can range from 1 to 15 cm [4].


Fig. 8.2
Grossly, collecting duct carcinomas are firm, white-grey tumors with ill-defined, infiltrative borders, often with multinodularity. Note the separate tumor nodules in the hilar fat
Microscopic Pathology
Histologically, CDC is essentially a high-grade poorly differentiated adenocarcinoma and most commonly shows tubules or tubulopapillary structures with irregular, angulated glands infiltrating the renal parenchyma (Fig. 8.3). However, other architectural patterns may be admixed in varying proportions, including solid cords, sheets, papillary formations with fibrovascular cores, a “hobnail” pattern, cystically dilated spaces, and cribriform histology [13]. At higher power, tumor cells have moderate to abundant eosinophilic cytoplasm with large hyperchromatic, pleomorphic nuclei and prominent nucleoli (Fig. 8.4) [2, 13]. As in other types of RCC, sarcomatoid differentiation and rhabdoid change can be seen (Fig. 8.5). Mitotic figures are frequently present. Both intraluminal and intracytoplasmic mucin may be seen, which can be highlighted with mucicarmine or Alcian blue stains. An additional characteristic feature of CDC is the presence of a pronounced desmoplastic stromal reaction, which can range in appearance from loose, myxoid, and collagenous to dense, eosinophilic, and fibrosclerotic. Associated inflammatory infiltrates, which are predominantly lymphocytic but occasionally mixed, are seen within the areas of desmoplasia (Fig. 8.6) [13]. Areas of geographic tumor necrosis and microscopic angiolymphatic invasion are also frequently present.
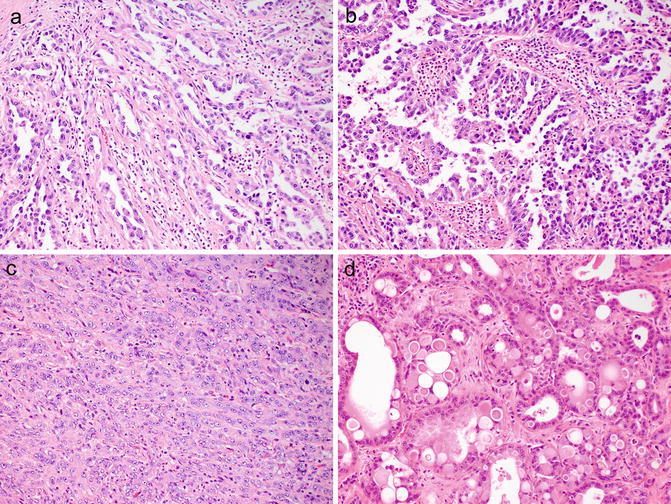
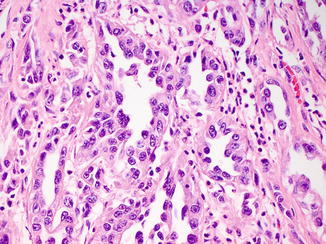
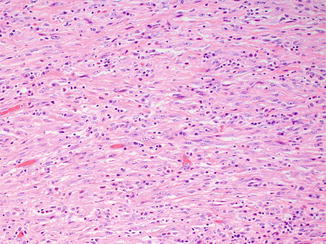
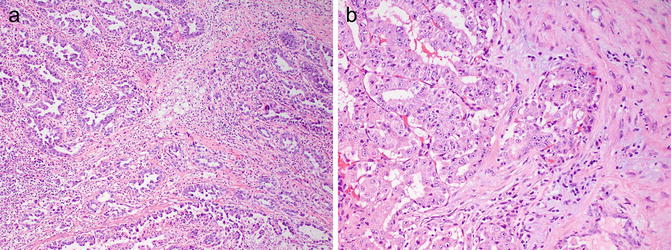

Fig. 8.3
Histologically, collecting duct carcinomas commonly show tubular (a) or tubulopapillary architecture with other admixed patterns, including papillary with true fibrovascular cores (b), solid cords and sheets (c), and cribriform (d)

Fig. 8.4
High-grade cytologic features in collecting duct carcinoma, including high nuclear to cytoplasmic ratio, nuclear enlargement and irregularity, pleomorphism, and prominent nucleoli

Fig. 8.5
Sarcomatoid differentiation as evidenced by high-grade, malignant spindle cells can also be seen in collecting duct carcinoma

Fig. 8.6
Pronounced desmoplastic stromal response in collecting duct carcinoma, which can be dense and fibrosclerotic (a) to myxoid (b). Associated lymphocytic inflammatory infiltrates are also present
Dysplasia of the adjacent renal tubular epithelium is another feature of CDC that can be helpful in making the diagnosis (Fig. 8.7), but can also occasionally be seen in urothelial carcinomas of the renal pelvis and in renal tubules adjacent to other types of RCC [7, 14]. Other more typical RCC patterns, urothelial carcinoma, or urothelial carcinoma in situ of the renal pelvis should not be present and would exclude the diagnosis of CDC [7].
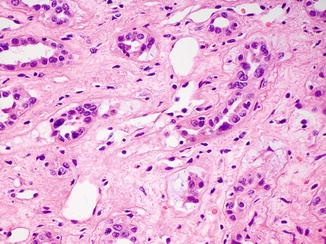

Fig. 8.7
Dysplastic tubules in areas adjacent to a collecting duct carcinoma can be a helpful diagnostic feature
Recently, the International Society of Urological Pathology (ISUP) has recommended the following histologic criteria for the diagnosis of CDC: (1) at least some of the lesion involves the medullary region; (2) there is a predominant formation of tubules; (3) a desmoplastic stromal reaction should be present; (4) cytologic features are high grade; (5) growth pattern is infiltrative; and (6) there is an absence of other typical RCC subtypes or urothelial carcinoma [15]. Because CDCs are by definition high-grade tumors, it has also been recommended that CDCs should not be assigned a grade (i.e., Fuhrman grade).
Immunohistochemistry
The immunohistochemical profile of CDC is reflective of the tumor’s origin from the cells of the collecting ducts in the renal medulla. Tumors are usually positive with lectins such as Ulex europaeus agglutinin-1 (UEA1) and peanut lectin. CDCs are also generally positive for low molecular weight cytokeratin (LMWCK), epithelial membrane antigen (EMA), PAX8, c-KIT/CD117, and vimentin, and a smaller number are positive for high molecular weight cytokeratin (HMWCK), CK7, CK20, and PAX2 [13, 16, 17]. In contrast, markers typically positive in proximal renal tubules such as CD10, alpha-methylacyl-CoA racemase (AMACR) , and RCC antigen are negative [18], as is p63, a com monly used marker of urothelial differentiation [17].
In contrast to RMCs, which show complete loss of staining for INI1 by immunohistochemistry (discussed below), only 15 % (3 of 20 cases) of CDC showed complete loss of INI1 staining in one study, and only 5 % (1 of 22 cases) in another study [19, 20]. Loss of INI1 sta ining in CDC does not portend a worse clinical outcome [19].
Cytogenetic and Molecular Findings
There are only limited data on the cytogenetic abnormalities seen in CDC, with no consistent genetic abnormality being identified to date. By conventional cytogenetics, CDCs show complex karyotypes with numerical and structural abnormalities involving multiple chromosomes [21–24]. More common abnormalities include loss of chromosomes 1, 13, 14, and 22. Loss of heterozygosity of 1q, 6p, 8p, 13q, and 21q by microsatellite analysis has also been reported in CDC [25, 26], while the characteristic loss of chromosome 3p commonly seen in clear cell RCC is not seen in CDC. Her2neu amplification was seen in about 50 % of CDC in a small series of cases [27].
Differential Diagnosis
The histologic differential diagnosis of CDC includes invasive urothelial carcinoma, papillary RCC, metastatic adenocarcinoma, unclassified RCC, and renal medullary carcinoma (summarized in Tables 8.2 and 8.3).
Table 8.2
Features helpful in distinguishing collecting duct carcinoma from other entities in the differential diagnosis
Feature | Collecting duct carcinoma | Urothelial carcinoma | Papillary renal cell carcinoma | Metastatic carcinoma |
|---|---|---|---|---|
Circumscription/encapsulation | No | No | Often both | Often circumscribed |
Focality | Unifocal | Unifocal | Unifocal | Multifocal |
Multinodularity | Often | Sometimes | No | No |
Desmoplastic stroma | Yes, prominent | Yes | No | Yes |
Other | Associated lymphocytic inflammation | Associated papillary lesion, urothelial CIS | Sometimes foam cells in papillary cores | Clinical history of other malignancies |
Immunohistochemistry | ||||
Positive | PAX8, UEA1 | p63, UEA1, CK20 | PAX8, CD10, RCC, AMACR | Possibly other lineage markers (e.g., TTF1, CDX2) |
Negative | p63, CK20 (rare +), CD10, RCC, AMACR | PAX8 | UEA1 | PAX8, RCC |
Table 8.3
Distinguishing histologic features in collecting duct carcinoma versus renal medullary carcinoma
Feature | Collecting duct carcinoma | Renal medullary carcinoma |
|---|---|---|
Dominant histologic pattern | Tubular, tubulopapillary architecture with angulated glands | Reticular composed of tubules, cords, and microcysts; irregular cribriform structures |
Inflammatory infiltrate | Predominantly lymphocytic to mixed | Predominantly neutrophilic to mixed |
Necrosis | Coagulative, geographic | Microabscess-like foci |
Sickled red blood cells in tissue | No | Yes |
Urothelial carcinoma involving the renal pelvis can be challenging to differentiate from CDC, especially if glandular differentiation and invasion of the renal parenchyma with desmoplasia are present. Identification of an associated urothelial papillary surface component, urothelial carcinoma in situ (CIS), squamous differentiation, or a predominance of other more typical patterns of urothelial carcinoma such as nested growth would essentially exclude the diagnosis of CDC [13, 18]. Immunohistochemistry can be helpful in this distinction, with PAX8 positivity seen in virtually all CDCs and only in 9 % of upper tract urothelial carcinomas [17]. Additionally, p63 positivity is seen in almost all upper tract urothelial carcinomas and in 14 % of CDCs. Further, when these two markers are interpreted together, the PAX8+/p63− profile gives a 100 % positive predictive value for CDC, and the PAX8−/p63+ profile gives a 100 % positive predictive value for urothelial carcinoma [17]. It should be noted that CK20 can be positive in a small number of CDC [13] and UEA1 is positive in both CDC and urothelial carcinoma [16]. Distinguishing between these two tumors has significant clinical implications, since CDC generally has an unfavorable prognosis, and patients with urothelial carcinoma generally require further evaluation of their urinary tract for additional urothelial lesions.
Papillary RCC may mimic CDC because of its predominant papillary or tubulopapillary architecture, especially if it is of high grade (papillary RCC, type 2). However, papillary RCCs are usually well circumscribed and encapsulated, in contrast to CDCs, which are grossly and histologically infiltrative. Papillary RCC also does not exhibit the desmoplastic stroma and angulated tubules of CDC. Immunohistochemistry can also distinguish between these two entities, as papillary RCC is usually positive for CD10, RCC antigen, and AMACR, while CDC is usually negative for these markers.
Metastatic adenocarcinoma , most commonly of colorectal or lung origin, is an important consideration in the differential diagnosis of CDC, as it also displays a marked desmoplastic stromal reaction. Generally, metastatic lesions tend to be multifocal, small, and relatively circumscribed. A previous history of malignancy would obviously be helpful in this distinction and could guide the selection of lineage-specific immunohistochemical markers such as TTF-1 and CDX2 to prove the metastatic nature of these lesions.
If a tumor of renal origin with infiltrative growth and desmoplasia is proven not to be urothelial or metastatic carcinoma and shows an absence of angulated glands with high-grade nuclear cytologic features, the diagnosis of unclassified RCC should be made [13]. Generally, these high-grade unclassified carcinomas are predominated by sheetlike, nested, and solid patterns. However, if there is any component of the tumor that meets the ISUP criteria for CDC (see above), it is recommended that the diagnosis should be “poorly differentiated CDC” and not “unclassified RCC ” [15].
Renal medullary carcinoma shows overlapping histologic features with CDC and is considered by some to represent an especially aggressive variant of CDC [7]. However, there are subtle features that can help differentiate between the two (summarized in Table 8.3; also see below for detailed description of RMC). First, CDC typically shows angulated tubules, glands, and tubulopapillary structures, while RMC commonly demonstrates a reticular pattern composed of anastomosing tubules and cords with irregular microcystic spaces. Both tumors show desmoplastic stroma with associated inflammatory infiltrates, but the inflammatory infiltrates in CDC are predominantly lymphocytic, in contrast to the neutrophilic to polymorphous infiltrates seen in RMC [13]. Additionally, CDC often shows areas of coagulative necrosis, while RMC will occasionally show distinctive microabscess-like areas of suppurative necrosis. Importantly, RMC clinically affects younger patients with sickle cell hemoglobinopathy who are commonly African American. Immunohistochemistry appears to have a limited role in distinguishing these two tumors since both are consistently positive for vimentin and UEA1 and variably positive for HMWCK, CK7, and PAX2 [13]. RMC consistently shows complete loss of immunohistochemical staining with INI1, but since a minority of CDC also shows this pattern of staining, it appears that INI1 immunohistochemistry cannot reliably distinguish these two tumors [15].
Renal Medullary Carcinoma
Gross Pathology
RMC shows a similar gross appearance to CDC, as these tumors are also white-grey to tan, firm to rubbery, poorly circumscribed with infiltrative borders, and centered in the renal medulla with variable hemorrhage and necrosis. There are often satellite nodules in the adjacent renal parenchyma corresponding to areas of lymphovascular invasion in large caliber vessels, as well as diffuse infiltration into the renal parenchyma, perinephric fat, or renal hilum [1, 7]. Tumors average 7 cm in diameter and can range from 4 to 18 cm [1, 28, 29]. Interestingly, RMC occurs more commonly in the right kidney (>75 %), but the reason for this is unclear [1, 18].
Microscopic Pathology
The most distinct and consistent histologic growth pattern seen in RMC is a reticular pattern formed by anastomosing tubules and cords with irregular microcytic spaces, imparting a resemblance to testicular yolk sac tumor (Fig. 8.8) [1, 13]. Often there is an admixture of architectural growth patterns including infiltrating cords, solid sheets, trabeculae, cribriform structures, and papillary with true fibrovascular cores [13]. Compact cribriform structures with rigid round spaces simulating adenoid cystic carcinoma can also be present. Tumor cells have moderate to abundant eosinophilic cytoplasm and high-grade nuclear features with prominent nucleoli. Areas demonstrating rhabdoid and sarcomatoid features may be present focally. Intracytoplasmic or intraluminal mucin is seen in a majority of cases.
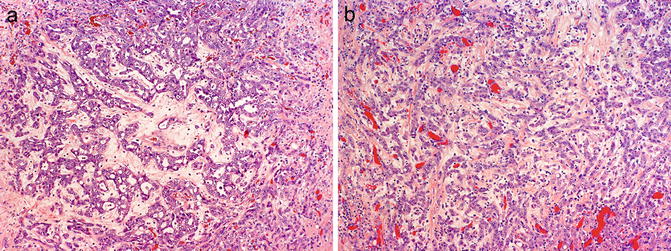

Fig. 8.8
Histologic patterns in renal medullary carcinoma include reticular with anastomosing cords, tubules, and microcystic spaces (a), infiltrating cords (b), solid nests, and cribriform. Note associated hypocellular, hyalinized desmoplastic stroma (photos provided by Dr. Liang Cheng, Indiana University)
A prominent desmoplastic stromal reaction is also a characteristic and tends to have a myxoid, edematous, hypocellular, loose, basophilic appearance. Focal areas with dense, eosinophilic, collagenous desmoplastic stroma are usually also appreciated. The associated inflammatory infiltrate can be quite striking and ranges from predominantly neutrophilic to polymorphous, including a mixture of neutrophils, lymphocytes, and eosinophils [7, 13]. Geographic and microabscess-like areas of necrosis can be seen.
As RMC almost invariably occurs in patients with sickle cell hemoglobinopathy, most commonly sickle cell trait, sickled red blood cells are frequently seen, both in the main tumor and within capillaries in the adjacent renal parenchyma (Fig. 8.9). In addition, the red blood cells may appear clustered or agglutinated within capillaries [14].
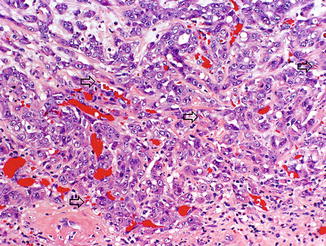

Fig. 8.9
Sickled red blood cells (arrows) are seen in capillaries of renal medullary carcinoma, as well as clusters of agglutinated red blood cells (photo provided by Dr. Liang Cheng, Indiana University)
Immunohistochemistry
By immunohistochemistry, RMC is consistently positive for cytokeratin AE1/AE3, LMWCK, EMA, vimentin, cytokeratin 7, CEA, and PAX8 [9, 13, 28–31]. Tumors also show variable positivity with HMWCK, cytokeratin 20, UEA-1, OCT3/4, and PAX2.
Complete loss of INI1/SMARCB1 expression in RMC by immunohistochemistry was first reported in 5 cases by Cheng et al. and has now been confirmed in 13 additional cases of RMC [20, 30] (details and significance discussed below). In RMC, INI1 is completely negative in tumor cells, while moderate to strong staining is present in inflammatory, stromal, and adjacent nonneoplastic collecting duct epithelial cells. Although this pattern of INI1 staining is not necessarily associated with rhabdoid cytologic features in RMC, most urothelial carcinomas and RCC are positive for immunohistochemical expression for INI1 even when rhabdoid features are present [31]. As mentioned above, a minority of CDC cases also show complete loss of INI1 expression. Thus, INI1 immunohistochemistry can be useful in distinguishing RMC from urothelial carcinomas and other RCCs, but is of more limited value in distinguishing RMC from CDC [15].
Cytogenetic and Molecular Findings
A small number of RMCs have been studied by conventional cytogenetics, which have generally shown complex karyotypes with a variety of insertions, deletions, and/or balanced translocations, but no recurrent genetic abnormalities have been identified [8]. Of note, one case showed the presence of a t(9;22) bcr-abl translocation, which was confirmed by fluorescence in situ hybridization (FISH) [32], and a subsequent study demonstrated ABL amplification in three tumors by FISH, but no bcr-abl translocation [5]. Comparative genomic hybridization analysis has shown an overall lack of genetic gains and losses in RMC, with only one case demonstrating loss of chromosome 22 [29].
Based on the results of immunohistochemistry studies, i t has been suggested that TP53, hypoxia inducible factor (HIF) , and vascular endothelial growth factor (VEGF) may play a role in the pathogenesis of RMC [29]. Molecular analysis of two RMC cases occurring in Caucasian patients without hemoglobinopathy has shown mutations in fumarate hydratase and von Hippel-Lindau (VHL) genes [8]. These findings suggest that a common underlying hypoxic cellular environment, either due to sickle cell trait/disease or mutations affecting hypoxia-sensing pathways, may subsequently lead to activation of HIF pathways and contribute to the development of RMC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








