9
Osteoporosis in Aging Men
Case History
An 80-year-old white man presented to the osteoporosis service 10 months after a fall with resultant L1 compression fracture. His past medical history was significant for two previous cerebrovascular accidents with secondary vascular dementia, seizure disorder, and balance disturbance. After his fracture, his primary care provider placed him on weekly alendronate therapy, which he was tolerating well. Additional significant history included Colles fracture at age 50, a height loss of approximately 3 inches since his mid-20s, poor erectile function, decreased energy level, and history of anticonvulsant therapy for 9 years.
Medications: Alendronate 70 mg weekly, Dilantin 200 mg daily, calcium 1500 mg daily, multivitamin tablet daily, atenolol 50 mg daily, Lescol 20 mg daily, quinine 325 mg daily
Social History: Married and living with wife; he has six children, all are alive and well; 100 pack per year smoking history, occasional beer on weekends
Family History: No known history of osteoporosis
Geriatric Review of Systems: Active; independent in activities of daily living; he ambulates with cane because of a fall 10 months ago, and is able to climb stairs and do yard work; he continues to drive.
Physical Exam: Height 64 inches; weight 116 lbs; body mass index (BMI) 20kg/m2
Cardiovascular System: Regular rate and rhythm; rate 70, BP 118/74
Neck: No thyromegaly
Back: Mild T-spine kyphosis; no pain on palpation of thoracic or lumbar spine
Genitalia: Testes 15mL bilaterally, soft
Laboratory:
Hemoglobin 11.6 g/dL, hematocrit 34.3%, mean corpuscular volume 107
Erythrocyte sedimentation rate 33 (0–15 mm/hr), serum protein electrophoresis within normal limits, thyroid-stimulating hormone (TSH) 0.8µU/mL, alkaline phosphatase 77
Creatinine 1.4mg/dL, ionized calcium 1.33mmol/L, (1.21–1.33 mmol/L), total calcium 9.8mg/dL (8.3–10.2), phosphorus 3.1mg/dL (3.0–4.8 mg/dL)
24-Hour Urine: Creatinine 0.86 g/d, calcium 0.072 g/d, phosphorus 0.143 g/d, creatinine clearance 43 cc/min
Parathyroid Hormone: Intact, 81 (11–54 pg/mL)
Total Testosterone (Total T): 220 ng/dL (200–1000 ng/dL)
25-Hydroxy Vitamin D: 32 ng/mL (15–65 ng/mL)
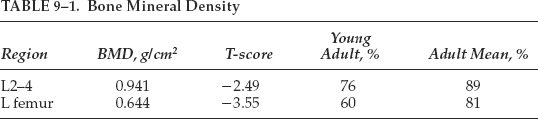
Bone Mineral Density: See Table 9–1
Osteoporosis is an often unrecognized but important concern in the care of elderly men. The impact of osteoporosis on the health of the male population must be considered in the context of falls, which significantly increase the risk for fracture. Although osteoporotic fractures are more common in women, 25 to 30% of all hip fractures in the United States and Northern Europe occur in men. In 1995, total medical costs associated with all osteoporotic fractures were estimated at $14 billion, and fractures in men accounted for $2.7 billion, or ∼20% of that sum.1 As a result of the increasing longevity of older persons, it is estimated that the number of hip fractures worldwide will increase from the present number, 1.7 million, to 6.3 million by 2050.2 Thus, prevention as well as diagnosis and treatment of osteoporosis are essential components in the comprehensive care of aging men.
Pathogenesis
Bone remodeling is a process that continues throughout life, long after maximum height and bone mineral density (BMD) are achieved. Bone remodeling represents a “coupling” of resorption of old bone with the formation or laying down of new bone. On the endosteal bone surfaces osteoclasts resorb bone, leaving a cavity. Osteoblasts then lay down osteoid in the cavity, and this new bone is subsequently mineralized. If the amount of bone replaced by the osteoblasts equals the amount originally removed by the osteoclasts, then there is no net bone loss. However, if more bone is resorbed than is formed, as is most common with aging, then irreversible bone loss occurs. Bone loss begins as early as the third decade in both men and women and is thought to result primarily from an early decrease in bone formation rather than from an increase in resorption. The factors responsible for this decrease in bone formation are not well understood. Bone loss later in life occurs primarily because of greater resorption relative to formation.3
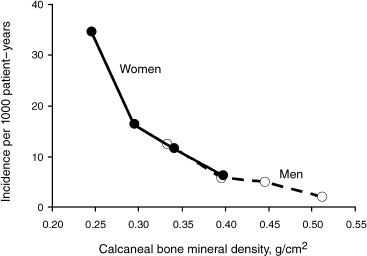
FIGURE 9-1. Incidence of new vertebral fractures per 1000 population per year as related to calcaneal bone mineral density. (Adapted from Melton LJ, Orwoll ES, Wasnich RD. Does bone density predict fractures comparably in men and women? Osteoporosis Int 2001;12:707–709, with permission.)
Determinants of Bone Mineral Density and Fracture Risk in Men
The relatively lower rate of osteoporosis in men compared with women is likely attributable to multiple factors, including larger bone size and thicker cortices in men, shorter male life expectancy, and lack of precipitous decline in sex hormone level analogous to the female menopause.4 At any given age, women are likely to have a lower BMD than age-matched men. For example, the mean femoral neck BMD for women age 60 to 69 is comparable to that of men over age 80.5 Figure 9–1 shows the differential fracture rates between men and women.
Several predictors of overall bone density in men have been identified. Orwoll et al6 studied 355 community-dwelling men (mean age 71.5 ± 7.4 years) and identified weight as one predictor of higher bone mass in multivariate analysis. Further, age, previous fracture, rheumatoid arthritis, gastrectomy, and hypertension were associated with lower bone mass.6 Bone mass and fracture risk have been strongly correlated in women, and studies suggest a similar relationship in men.4,7
Diagnosis of Osteoporosis in Men
In 1993 the Osteoporosis Consensus Development Conference in Hong Kong defined osteoporosis as “a metabolic bone disease characterized by low bone mass and microarchitectural deterioration of bone tissue leading to enhanced bone fragility and a consequent increase in fracture risk.” By correlating BMD with fracture risk, the World Health Organization (WHO) determined BMD values indicative of osteoporosis specifically in reference to postmenopausal Caucasian women. As BMD values have not yet been specifically validated by correlation with fracture risk in men, the WHO criteria for diagnosing osteoporosis in women are applied to men as well.
Application of the WHO criteria for women to the male population introduces inaccuracies. Faulkner and Orwoll8 used multiple BMD measurement techniques that resulted in varied estimates of osteoporosis prevalence that may not accurately identify those men at risk for fracture. Further, these authors found that the established female diagnostic criteria of a T-score of ≤ –2.5 underestimates osteoporosis prevalence in men when comparing prevalence with fracture risk estimates.8 Although the WHO criteria are imperfect, there are no other currently accepted criteria in men and, consequently, these criteria are routinely applied to men. Further study is needed to establish diagnostic criteria for osteoporosis specific to the male population, and a longitudinal study is now ongoing.
Screening for Osteoporosis in the Male Patient
Currently, there are no national guidelines for screening of osteoporosis in men. Burgess and Nanes9 recommend screening with hip/spine dual-energy x-ray absorptiometry (DEXA) for asymptomatic men beginning at age 70 unless risk factors are present. They recommend screening men at age 55 if risk factors are identified, including previous fracture, current or past smoking history, family history of osteoporotic fracture, body mass index less than 18kg/m2, or prior glucocorticoid use. Men of any age on glucocorticoids should be screened and receive calcium plus vitamin D supplementation.
In our own geriatrics clinic, a careful history is taken in all men to assess osteoporosis risk factors. Ideally, all men age 70 and over would receive a DEXA scan to determine their risk for future fracture. However, the issue of payment is a major obstacle to general screening for the population of older men. If osteoporotic risk factors exist or a history of a nontraumatic fracture is present, then Medicare will cover the cost of a DEXA in those men. If DEXA is performed, results guide the next step in management. If the initial DEXA is normal (T ≫ −1.0), then patient education is provided addressing adequate calcium plus vitamin D intake and exercise recommendations. DEXA may be repeated in 3 to 5 years. If the DEXA reveals osteopenia (T= −1.0 to −2.5), calcium plus vitamin D intake and exercise recommendations are discussed, and consideration is given to pharmacological management, particularly if risk factors are present. When osteoporosis is present on DEXA (T < −2.5), we evaluate for secondary causes, and guide treatment from these results. If an underlying cause is identified, then that must be treated first, and subsequently pharmacological therapy for osteoporosis should be considered. Patients with history of a fragility fracture resulting from minimal or no trauma are defined as osteoporotic regardless of BMD, and evaluation for secondary causes and pharmacological management is advised.
Differentiating Osteoporosis and Osteomalacia
The distinction between osteoporosis and osteomalacia is important, although both disorders may coexist. Osteomalacia is a histomorphometric diagnosis that is defined as a defect in bone mineralization and may appear as bone loss on bone density measurement.10 Bone resorption may increase, and pseudofractures can develop. Vitamin D deficiency in the elderly may be attributable to poor nutrition, lack of exposure to sunlight, malabsorptive disorders, and decreased ability of the skin to convert 7-dehydrocholesterol to cholecalciferol (vitamin D3) by ultraviolet radiation.10,11 Laboratory evaluation in osteomalacia reveals low 25-hydroxy vitamin D, low or normal serum calcium, low serum phosphorus, elevated alkaline phosphatase, and increased parathyroid hormone (PTH) levels.10 Bone biopsy confirms the diagnosis, but is generally not required. If weight loss and/or symptoms of malabsorption are present, evaluation for celiac sprue or other malabsorption syndromes is indicated. Mild vitamin D deficiency can be associated with osteoporosis, and vitamin D is prescribed with the goal of increasing calcium absorption from the gut, as well as stimulating bone formation and mineralization.10 Treatment consists of pharmacological doses of vitamin D administered orally, beginning with 50,000 IU weekly for 4 weeks followed by daily supplementation with 400 to 800 IU. Alternatively, intramuscular injections may be used, specifically in cases where absorption by mouth is poor or inadequate; however, the intramuscular formulation is no longer available.
Secondary Causes of Osteoporosis
A review by Taxel and Kenny12 reported an incidence of secondary causes in osteoporotic men ranging from 50 to 80%, with steroid therapy and hypogonadism accounting for 13 to 17% and 14 to 16%, respectively. Secondary causes are numerous and are listed in Table 9–2. Some of the more common etiologies are discussed in the following sections. Endocrine disorders are common in the aging population and should routinely be considered in the evaluation of secondary osteoporosis.
Primary Hyperparathyroidism
Primary hyperparathyroidism is defined biochemically as elevated serum calcium (ionized or total) with an inappropriately elevated PTH level. A common mistake is failure to diagnose primary hyperparathyroidism when an elevated calcium level is measured along with a PTH in the normal range. The “normal” PTH lvelel is inappropriate for the level of calcium (should actually be suppressed or at the low end of the normal range), and primary hyperparathyroidism is likely present.
Hyperparathyroidism dramatically impacts bone mineral density, resulting in selective cortical bone loss. The relationship between bone loss and PTH is incompletely understood: chronic, continuous excess PTH results in cortical bone loss; however, intermittent, exogenous PTH administration increases trabecular bone mass with minimal impact on cortical bone.13 Two retrospective cohort studies suggest that overall risk of fracture is increased in patients with primary hyperparathyroidism.14
Management options in mild hyperparathyroidism include observation for asymptomatic patients, surgery, and medical management. Observation of patients with asymptomatic disease is a reasonable approach, and should be accompanied by biannual to annual surveillance for symptoms and laboratory criteria for surgery. The National Institute of Health Consensus Conference held in April 2002 issued guidelines for surgical intervention in primary hyperparathyroidism.15 These recommendations advise surgery if serum calcium is greater than 1mg/dL above the upper limits of normal; 24-hour urinary calcium is greater than 400 mg; creatinine clearance is reduced by more than 30% compared with age-matched subjects; BMD at the lumbar spine, hip, or distal radius is more than 2.5 standard deviations below peak bone mass; the patient is under 50 years of age; or when medical surveillance is either not desirable or not possible for an individual patient.15 Studies reveal a 10 to 15% increase in bone mass following surgical management of primary hyperparathyroidism.13 In studies of postmenopausal women with primary hyperparathyroidism, medical management via hormone replacement therapy (HRT) with estrogen resulted in decreased bone loss.13 However, due to adverse events associated with HRT in the Women’s Health Initiative trial, this is no longer a recommended treatment. Bisphosphonates offer another option for medical management of hyperparathyroidism. In one trial of 27 women and five men, subjects treated with alendronate for 2 years showed significant gains in bone density at the lumbar spine relative to controls.16
| Endocrine Disorders Primary/secondary hypogonadism Endogenous/exogenous glucocorticoid excess Hyperparathyroidism Hyperthyroidism Hyperprolactinemia Diabetes mellitus type I |
| Malignancy and Disorders of the Bone Marrow Systemic mastocytosis Multiple myeloma Lymphoproliferative diseases Myeloproliferative diseases |
| Gastrointestinal Disorders Primary biliary cirrhosis Inflammatory bowel diseases Celiac sprue Postgastrectomy |
| Connective Tissue Disorders Rheumatoid arthritis Osteogenous imperfecta Ankylosing spondylitis |
| Other Disorders Chronic obstructive pulmonary disease Homocystinuria Hemochromatosis Anorexia nervosa |
| Medications Glucocorticoids Heparin Cyclosporin A Warfarin Thyroid hormone/in excess Antiepileptics Chemotherapy causing chemical castration LHRH agonists Lithium Methotrexate |
Adapted from Harper KD, Weber TJ. Secondary osteoporosis: diagnostic consideration. Endocrinol Metab Clin North Am 1998;27:325–348, with permission.
Finally, the recently cloned extracellular calcium-sensing receptor, which plays a central role in calcium homeostasis, represents an important target for therapeutic agents aimed at treating disorders of calcium metabolism. Compounds known as calcimimetics act as agonists of calcium at this receptor and can lower PTH and calcium concentrations. These agents hold promise as potential medical treatments for diseases of parathyroid hyperfunction, such as primary and secondary hyperparathyroidism.17
Thyroid Dysfunction
Long-standing, untreated hyperthyroidism leads to decreased bone density and confers an increased risk of osteoporotic fracture.13 In patients with hypothyroidism, BMD generally remains normal and long-term replacement of thyroxine causes only minimal loss of BMD in the spine.13
Gonadal and Pituitary Dysfunction
It is well established that severe testosterone deficiency (hypogonadism) leads to significant bone loss. However, the gradual decline in testosterone (T) with age in men has not been definitively established as a cause of osteoporosis.18 Studies of men over 65 years of age receiving testosterone replacement have demonstrated inconsistent results. In one randomized controlled study of testosterone replacement in 44 hypogonadal men age 65 and older, BMD was maintained in testosterone-treated subjects over 12 months (Fig. 9–2).19 Other studies have shown testosterone replacement to improve BMD in selected men with total testosterone levels below 200 ng/dL.20 A randomized, double-blinded, placebo-controlled study of 96 men over 65 years of age showed testosterone treatment to have minimal impact on BMD in men with pretreatment serum testosterone concentrations ≥400ng/mL, but BMD did improve in those subjects with pretreatment serum testosterone concentrations ≤200ng/dL.21 These results suggest that a serum testosterone level of 200ng/dL may be a critical value below which osteoporosis risk increases, and consequently testosterone therapy may be beneficial under such circumstances.22 Studies to evaluate the impact of testosterone replacement on fractures risk have not been reported.20
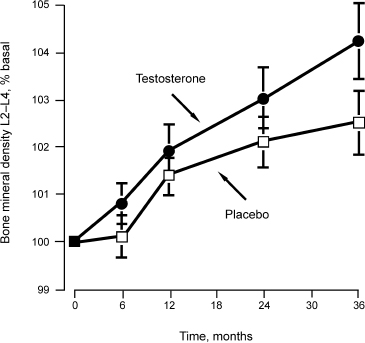
FIGURE 9-2 The effects of testosterone versus placebo in restoring bone mineral density over time.
Recent “experiments of nature” have demonstrated the importance of 17-β-estradiol (E2) in the acquisition of peak bone mass in men. Individuals with genetic defects in the estrogen receptor or in aromatase,23–25 the enzyme that converts androgens to estrogens,26 have demonstrated osteoporosis and increased bone turnover. Because all estrogens in men are derived from androgens through the aromatase pathway, defects in this enzyme lead to extremely low E2 levels. A patient with an aromatase defect gained significant bone mass after the institution of E2 therapy27 Several observational studies have demonstrated that serum E2 levels are better predictors of BMD in older men than serum T levels.28 In older men in whom both gonadotrophin secretion and aromatase conversion were suppressed, Falahati et al29 demonstrated in a short-term study that E2 is the principal sex steroid regulating bone resorption. Thus, current evidence suggests an important role for estrogen in the pathogenesis of osteoporosis in men, and future research will help further clarify this role.
Growth hormone is now understood to play a role in maintenance of skeletal mass. Two studies evaluated growth hormone supplementation in growth-hormone-deficient adults and revealed improved BMD at the total body, hip, and spine. Research to determine if growth hormone administered to elderly patients can improve BMD or reduce fractures has not yet been completed.13
Gastrointestinal Disorders
Several gastrointestinal disorders have been associated with low bone mass. Osteopenia and fractures are more common in patients with primary biliary cirrhosis.10 Patients with a history of gastrectomy have lower BMD and a higher rate of both femoral and vertebral fractures.18 Calcium malabsorption leads to secondary hyperparathyroidism and, subsequently, to osteoporosis. Patients with mild malabsorption may have markedly low BMD. In one study, antigliadin antibodies were detected in 12% of patients with idiopathic osteoporosis and in 3% of healthy age-matched controls.18 The incidence of biopsy confirmed sprue was 10 times higher in osteoporotic patients relative to controls.18 Crohn’s disease with involvement of the proximal small bowel leads to malabsorption of calcium and subsequent development of osteoporosis.
Malignancies
In malignant monoclonal gammopathy, plasma cells produce osteoclast-stimulating cytokines resulting in the uncoupling of resorption and formation with the ultimate development of osteoporosis.18
Rheumatic Diseases
Periarticular and generalized osteoporosis are associated with rheumatoid arthritis (RA), independent of the effect of medications used to manage this entity. Spine and hip BMD are decreased and have been correlated with cumulative corticosteroid doses. Patients with RA who have not received corticosteroid therapy also exhibit decreased bone mineral content at the distal radius that correlates with the duration of disease.18
Medications
Exogenous glucocorticoid excess is the most common cause of secondary osteoporosis and is a function of dose and duration of therapy. Glucocorticoid-induced osteoporosis is due to a combination of increased bone resorption and decreased bone formation. Secondary hyperparathyroidism and hypogonadism may play a role in resorption. Decreased bone formation is probably a direct effect of inhibition of osteoblast function.18 The doses of glucocorticoids that produce osteoporosis are not well defined. More than 7.5 mg/day of prednisone or its equivalent is likely to cause bone loss, but lower doses in patients who have additional risk factors may lead to bone loss. The lowest dose and the lowest frequency of administration that are effective for the patient should be used.
The anticonvulsants diphenylhydantoin and phenobarbital can be important factors in bone loss due to their role in vitamin D metabolism. Although exact mechanisms are not thoroughly elucidated, these agents augment metabolism of vitamin D. Pharmacological doses of vitamin D supplementation may be required to maintain acceptable levels.
Long-term heparin therapy leads to osteoporosis, though some studies suggest low molecular weight heparin may cause less bone loss than unfractionated heparin.18 The immunosuppressant cyclosporin A and glucocorticoids are administered after organ transplantation, and rapid decreases in bone density may occur, though the degree of bone loss attributable to cyclosporin A alone is difficult to determine.18 Chronic methotrexate therapy leads to bone pain, swelling, and fractures.18
Luteinizing hormone–releasing hormone (LHRH) agonist therapy for prostate cancer was shown in two prospective studies to cause bone loss and increases risk of osteoporotic fractures.30,31 This is primarily due to extreme lowering of both testosterone and estrogen by these agents.
Smoking and Alcohol
Epidemiological twin studies suggest a correlation between smoking and peak adult bone mass.18 In a cohort study of men, smoking decreased bone mass; however, no research has determined the direct impact of smoking on fractures.18 Conversely, chronic alcoholism has been shown to increase fracture rate.18 Mild to moderate intake of alcohol has not been associated with bone loss.
Laboratory Evaluation
A directed algorithm for laboratory workup of secondary causes of osteoporosis is presented in Fig. 9–3.10 Due to the high incidence of secondary causes of osteoporosis in men, all men diagnosed with osteoporosis should be evaluated for secondary causes, and this scheme provides a systematic approach to this evaluation. Initial laboratory studies should include measurement of calcium and creatinine in blood and urine, thyroid function screening with TSH, protein electrophoresis, total testosterone, phosphorus, and a complete blood count. If initial screening reveals abnormalities, further measurements of calciotropic hormones (PTH and 25-hydroxy vitamin D) and serum and urine phosphorus should be performed. If total T is < 200ng/dL, then follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are obtained to rule out pituitary or hypothalamic disease. If these are normal or low [inappropriate to the level of testosterone (T)], then magnetic resonance imaging (MRI) is indicated and consultation with an endocrinologist may be warranted. If T is between 200 and 400 ng/dL, then we repeat total T, free T, and sex hormone-binding globulin (SHBG) to determine gonadal status. Due to the normal diurnal variation in T levels, more than one laboratory assessment of T level is often required. Careful history and physical examination should direct additional testing such as tests of gastrointestinal and renal function to diagnosis specific secondary causes. The clinician should maintain a high clinical suspicion for secondary causes as these are frequently missed.10
Nonpharmacological Treatment of Osteoporosis in the Men
Patient education is an important component of osteoporosis management. Education should include information about nonpharmacological measures. Regular weight-bearing exercise as well as smoking cessation and falls prevention should be discussed.
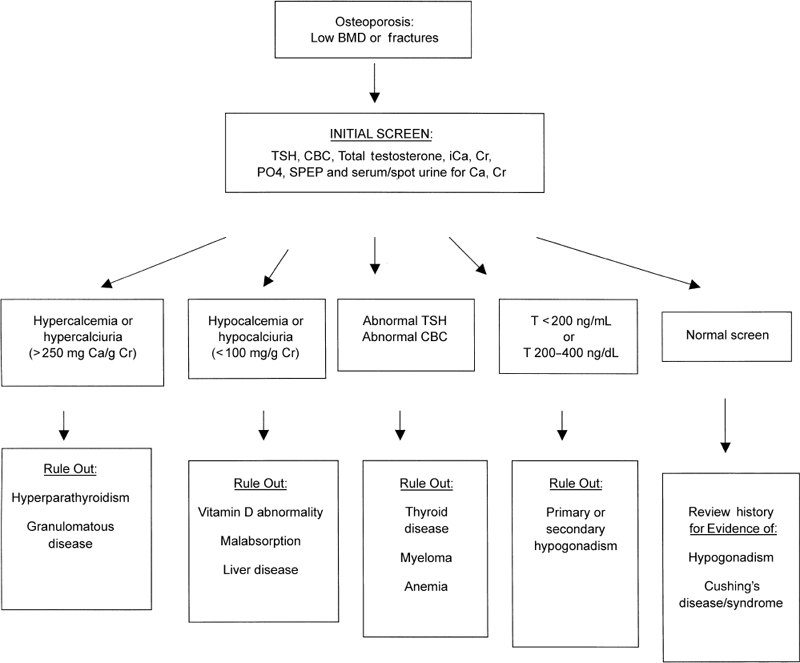
FIGURE 9-3. Algorithm for laboratory work-up for secondary causes of osteoporosis. Ca, calcium; CBC, complete blood count; Cr, creatinine; iCa, ionized calcium; PO4, phosphorus; SPEP, serum protein electrophoresis; TSH, thyroidstimulating hormone. (Adapted from Taxel P, Kenny A. Differential diagnosis and secondary causes of osteoporosis. Clin Cornerstone 2000;2:11–21, with permission.)
Falls
Falls in the geriatric population have serious public health implications, both for the individual and for society. In one study of 1103 community-dwelling men and women aged 72 and older, 49% of the cohort experienced at least one fall during a 1-year follow-up period.32 Serious injury, including fracture, joint dislocation, other serious joint injury, serious laceration, and intracranial injury, occurred in 23% of those falls.32 Additional consequences of falls include minor injuries, inability to get up after a fall, and fear of future falls with resultant decreased activity.33 From an economic perspective, in 1999 injuries among Medicare fee-for-service beneficiaries cost the system over $8 billion.34 Fractures comprised 67% of injuries and cost over $5 billion.34 Multiple studies have identified risk factors for falls in the elderly.35 Modifiable risk factors for falls include mobility and transfer impairment, balance disturbance, multiple medications, sensory and perceptive deficits, postural hypotension and dizziness, foot and footwear problems, and environmental hazards. Identification of these risk factors should prompt subsequent intervention to reduce the probability of falls. According to the Cochrane Data-base of Systematic Reviews, hip protectors reduce the risk of hip fracture when used in a selected, high-risk population.36 Cost effectiveness was not determined.36 Patient compliance was an issue secondary to discomfort and practicality.36 Nonetheless, hip protectors are a low-risk intervention and should be discussed with patients who are at high risk for osteoporotic hip fracture (Fig. 9–4). Specific environmental hazards contributing to the risk of falls include throw rugs in the home, inadequate lighting, and extension cords traversing floors in walking areas. Hence, patient education is an important adjunct to pharmacological management of osteoporosis.

FIGURE 9-4. Hip protectors (B and C) inserted beneath underwear (A) may prevent hip fractures in patients at high risk.
Pharmacological Treatment of Osteoporosis in the Men
Pharmacological management should include calcium supplementation of 1.2 g/day and vitamin D supplementation of 400 to 800 IU/day.9 Calcium can be obtained from food sources and in combination with calcium supplements that are available over the counter. When calcium supplements are used, we warn the older patient of side effects such as constipation, gas, or bloating. These effects may be remedied by switching brands or increasing fiber in the diet. Calcium carbonate requires stomach acid for absorption; therefore, it is best taken with meals. It should be avoided in patients with achlorhydria. Calcium citrate can be taken any time during the day or evening and does not require an acidic stomach pH. There are minimal data thus far to document that one calcium salt is superior to another.
Vitamin D is found in several food sources including milk, in combination with calcium in supplements or in multivitamins. We recommend 400 to 800IU per day, and we check vitamin D levels in most of our older patients.
Bisphosphonates
Alendronate is Food and Drug Administration (FDA) approved for use specifically in men with osteoporosis. A double-blind placebo-controlled trial of 241 men (mean age 63) found alendronate significantly increases BMD for the spine, hip, and total body, as well as prevents vertebral fractures.37 The effect was significant in men with and without hypogonadism. Alendronate and risedronate are FDA approved for both prevention and treatment of glucocorticoid-induced osteoporosis. Alendronate and risedronate can be dosed at 70mg weekly and 35 mg weekly, respectively. The most commonly reported side effects of bisphosphonates are gastrointestinal and include esophagitis, abdominal pain, dyspepsia, nausea, vomiting, and diarrhea. Muscle pain has also been reported. Bisphosphonates should be avoided in patients with a history of significant gastroesophageal reflux, gastritis, or peptic ulcer disease. Patients should be instructed to take bisphosphonates upon rising in the morning with an 8-ounce glass of water; they should remain upright (sitting or standing) for a half-hour after taking these medications and should refrain from eating or drinking anything, as well as taking other medications, during that time.
Teriparatide (Human Parathyroid Hormone 1–34)
Teriparatide (human PTH) was recently FDA approved for treatment of osteoporosis in men. PTH is indicated to increase BMD in men with primary or hypogonadal osteoporosis who have high fracture risk, including those with history of osteoporotic fracture, those with multiple risk factors for fracture, and those who are failed or did not tolerate alternative therapies. In a randomized, placebo-controlled trial of 437 men with spine or hip BMD greater than 2 standard deviations below the young adult male mean, PTH was evaluated at a dose of 20 and 40 µg/day.38 In this study, PTH significantly increased the bone mineral density of men for the spine, femoral neck, and whole body; radial bone mineral density did not change. Response to PTH was similar regardless of gonadal status, age, baseline BMD, BMI, smoking, or alcohol consumption.38 PTH has been shown to reduce fracture rates in women, and recent data in men demonstrate a significant decrease in vertebral fracture.39 Reported side effects include local injection site reactions, headaches, nausea, arthralgias, and hypercalcemia. Studies using PTH were terminated early due to findings of osteosarcoma in rats. Long-term safety and efficacy data on PTH are limited, and therefore the recommended duration of use is 2 years or less with subsequent antiresorptive therapy for maintenance of BMD.
Case Summary and Conclusion
The patient cited previously presented with an L1 vertebral compression fracture secondary to a fall from standing height, and therefore meets the criteria for severe osteoporosis. He takes adequate calcium and vitamin D in his diet (normal serum vitamin D level) and actively exercises, which is the base of any osteoporosis program. Because the total testosterone falls between 200 and 400 ng/dL, our next step would be to perform repeat laboratory evaluation to include a total and free T (or bioavailable) and SHBG. If these are low (total and free T), then the follicle-stimulating hormone (FSH) and LH are checked to rule out primary or secondary hypogonadism. If total and free T are low with normal SHBG, but elevated FSH and LH, the diagnosis is consistent with primary hypogonadism, a known risk factor for secondary osteoporosis. If T is low (either total or free) with a low or normal FSH and LH (inappropriate), then a further endocrine evaluation including MRI may be warranted. T replacement therapy would be important here not only for bone health and erectile function (which may or may not be improved on T replacement) but also for muscle mass, energy, and sense of well-being. Because there is no present evidence that T replacement decreases fractures, we would also recommend therapy with an oral bisphosphonate, if the patient was able to tolerate it. Long-standing anticonvulsant therapy with Dilantin may also be a contributor to this patient’s bone loss, and a change of seizure medication that doesn’t interfere with vitamin D metabolism could be considered. The patient also has a high-normal ionized calcium level with a normal total calcium and an elevated PTH, which may represent primary hyperparathyroidism. I would repeat these measures to verify and also include an albumin to adjust total calcium level. He has a low BMD, but otherwise does not meet the criteria for surgical intervention. We would likely opt for treatment with antiresorptive therapy to improve bone mass in patients with primary hyperparathyroidism. Finally, a repeat bone density test should be performed at 12 to 18 months to determine if therapy is stabilizing bone loss or increasing bone mass.
References
1. Ringe JD, Orwoll E, Daifotis A, Lombardi A. Treatment of male osteoporosis: recent advances with alendronate. Osteoporos Int 2002;13:195–199
2. Seeman E. Unresolved issues in osteoporosis in men. Rev Endocr Metab Disord 2001;2:45–64
3. Seeman E. Pathogenesis of bone fragility in women and men. Lancet 2002;359:1841–1850
4. Kenny A, Taxel P. Osteoporosis in older men. Clin Cornerstone 2000;2:45–51
5. Melton LJ, Orwoll ES, Wasnich RD. Does bone density predict fractures comparably in men and women? Osteoporos Int 2001;12:707–709
6. Orwoll ES, Bevan L, Phipps KR. Determinants of bone mineral density in older men. Osteoporos Int 2000;11:815–821
7. Melton LJ, Atkinson EJ, O’Connor MK, O’Fallon WM, Riggs BL. Bone density and fracture risk in men. J Bone Miner Res 1998;13:1915–1923
8. Faulkner K, Orwoll E. Implications in the use of T-scores for the diagnosis of osteoporosis in men. J Clin Densitom 2002;5:87–93
9. Burgess E, Nanes MS. Osteoporosis in men: pathophysiology, evaluation, and therapy. Curr Opin Rheumatol 2002;14:421–428
10. Taxel P, Kenny A. Differential diagnosis and secondary causes of osteoporosis. Clin Cornerstone 2000;2:11–21
11. Cobbs EL, Duthie EH, Murphy JB, eds. Geriatrics Review Syllabus: A Core Curriculum in Geriatric Medicine. 5th ed. Madlen, MA: Blakwell Publishing for the American Geriatrics Society; 2002:182
12. Taxel P, Kenny A. Differential diagnosis and secondary causes of osteoporosis. Clin Cornerstone 2000;2:11–21
13. Rosen CJ. Endocrine disorders and osteoporosis. Curr Opin Rheumatol 1997;9:355–361
14. Khosla S, Melton LJ. Fracture risk in primary hyperparathyroidism. J Bone Miner Res 2002;17:N103–N107
15. Bilezikian JP, Potts JT, Fuleihan GE, et al. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab 2002;87:5353–5361
16. Parker CR, Blackwell PJ, Fairbairn KJ, Hosking DJ. Alendronate in the treatment of primary hyperparathyroid-related osteoporosis: a 2-year study. J Clin Endocrinol Metab 2002;87:4482–4489
17. Cohen A, Silverberg SJ. Calcimimetics: therapeutic potential in hyperparathyroidism. Curr Opin Pharmacol 2002;2:734–739.
18. Harper KD, Weber TJ. Secondary osteoporosis: diagnostic consideration. Endocrinol Metab Clin North Am 1998;27:325–348.
19. Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci 2001;56:M266–M272
20. Iannuzzi-Sucich M, Kenny AM. Osteoporosis in older men. In: Martini L, ed. Encyclopedia of Endocrine Diseases, Vol. 3. San Diego: Academic Press; 2004:432–435
21. Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab 1999;84:1966–1972
22. Yialamas MA, Hayes FJ. Androgens and the aging male. Endocrinol Rounds 2003;2.
23. Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 1994;331:1056–1061
24. Morishma A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 1995;80:3689–3698
25. Carani C, Qin K, Simoni M, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 1997;337:91–95
26. Simpson E, Rubin G, Clyne C, et al. Local estrogen biosynthesis in males and females. Endocr Relat Cancer 1999;6: 131–137
27. Bilezekian J, Morishima A, Bell J, Grumbach MM. Estrogen markedly increases bone mass in an estrogen deficient young man with aromatase deficiency N Engl J Med 1998;339:599–603
28. Riggs LB, Khosla S, Melton J. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res 1998;13:763–773
29. Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 2000;106:1553–1560
30. Daniell HW, Dunn SR, Ferguson DW, et al. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol 2000;163:181–186
31. Diamond T, Campbell J, Bryant C, et al. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma. Cancer 1998;83:1561–1566
32. Tinetti ME, Doucette J, Claus E, Marottoli R. Risk factors for serious injury during falls by older persons in the community. J Am Geriatr Soc 1995;43:1214–1221
33. King MB, Tinetti ME. Falls in community-dwelling older persons. J Am Geriatr Soc 1995;43:1146–1154
34. Bishop CE, Gilden D, Blom J, et al. Medicare spending for injured elders: are there opportunities for savings? Health Aff (Millwood) 2002;21:215–223
35. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988;319):1701–1707
36. Parker MJ, Gillespie LD, Gillespie WJ. Hip protectors for preventing hip fractures in the elderly. The Cochrane Database of Systematic Reviews 2003;3:CD0001255
37. Orwoll E, Ettinger M, Weiss S, et al. Alentronate for the treatment of osteoporosis in men. N Engl J Med 2000;343:604–610
38. Orwoll ES, Scheele WH, Paul S, et al. The Effect of triparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 2003;18:9–17
39. Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporosis Int 2004. Online publication
< div class='tao-gold-member'>