Fig. 3.1
Gastric blood supply and preparation of the stomach for resection and gastric conduit creation
Indications for Transhiatal Esophagectomy
Indications for transhiatal esophagectomy have been previously reported by Orringer et al. [7]. Esophagectomy may be performed for benign disease, including Barrett’s mucosa with high-grade dysplasia, achalasia, reflux stricture, recurrent hiatal hernia, recurrent gastroesophageal reflux, spasm/dysmotility, acute perforation, and caustic stricture. Esophagectomy for carcinoma may also be performed for any histology and also for disease at any level of the esophagus.
Preoperative Details
The patient is instructed to perform aggressive outpatient pulmonary physiotherapy with an incentive spirometer and daily exercise. Smoking must be stopped at least 2 weeks before surgery. In patients with documented or suspected chronic pulmonary disease we obtain formal pulmonary function testing and arterial blood gas measurements. When adequate oral intake is not possible, a nasogastric feeding tube is placed and outpatient enteral nutrition is administered. If the stomach may not be a suitable conduit then a barium enema is performed to evaluate the colon as a potential conduit, and standard bowel prep is administered for possible colon interposition. In patients receiving neoadjuvant chemotherapy and radiation, we schedule the operation 4 weeks after the last treatment, but any time between 3 and 5 weeks after completion of neoadjuvant therapy is reasonable. The development of severe and possibly prohibitive radiation fibrosis occurs as early as 6 weeks post-radiation treatment.
Surgical Technique
Patient Positioning
The patient is positioned supine with arms tucked and padded at the sides and a shoulder roll under the scapula to extend the neck. If the patient had a feeding gastrostomy or jejunostomy tube placed preoperatively, this is removed and the skin is sutured closed. The head is turned to the patient’s right and the entire neck, chest, and abdomen are prepped from the mandible to the symphysis pubis (Fig. 3.2).
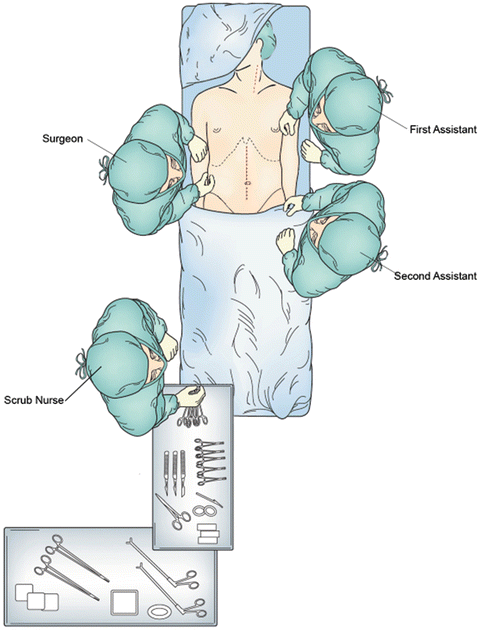

Fig. 3.2
Patient positioning and operating room setup
Mobilization of the Gastric Conduit
The abdominal portion of the operation is started first through a supra-umbilical laparotomy. The triangular ligament of the liver is taken down and an assessment of the abdominal cavity is performed to rule out metastatic disease and to ascertain the suitability of the stomach as a conduit. The right gastroepiploic vessels are identified and protected and the lesser sac is entered through an avascular portion of the greater omentum. The short gastric vessels high up on the greater curvature are divided close to the stomach (Fig. 3.1). The omentum is separated from the right gastroepiploic artery with a 1.5 cm distance away from the vessel to the level of the pylorus. The peritoneum over the esophageal hiatus is divided and the esophagus and its overlying fat pad are dissected free from the hiatus. The gastrohepatic omentum is divided and the left gastric vessels are isolated and divided, which can be accomplished by suture ligation or vascular staplers (Fig. 3.1). If an aberrant left hepatic artery is encountered in the gastrohepatic omentum, efforts should be made to preserve this vessel if possible. Gastrohepatic and left gastric artery lymph nodes should be included with the gastric specimen. Separate celiac axis lymph nodes should be sent in the setting of cancer resection. It is not typically necessary to divide the right gastric vessels and attempts should be made to preserve them.
Drainage Procedure
A Kocher maneuver is performed to allow the pylorus to reach approximately the level of the xiphoid process. Occasionally, the pylorus can reach the xiphoid process without a Kocher maneuver. A gastric emptying procedure is then performed, classically a pyloromyotomy beginning 1.5 cm on the gastric side and continuing for approximately 1 cm onto the duodenum. Needle-tip electrocautery is particularly useful for this maneuver with care taken to avoid tears in the mucosa. Mucosal injuries should be repaired using 4-0 or smaller suture. Clips can be placed near the pylorus for future radiographic identification of the location of the pylorus during gastric emptying studies. Alternatively, several authors have reported excellent results using botulinum toxin injected into the pylorus instead of performing pyloromyotomy. However, long-term results and comparative results against other drainage techniques are not yet available for the botulinum toxin approach [8, 9].
Initial Mediastinal Dissection
At the diaphragmatic hiatus, a narrow Deaver retractor is placed into the hiatus for additional exposure. The lymph nodes in the peri-esophageal fat around the distal esophagus are dissected towards the esophagus to be included with the specimen. Entry into one or both pleural spaces frequently occurs during this step. We routinely place bilateral chest tubes at the beginning of surgery due to the potential for unrecognized entry into the pleural spaces and subsequent development of symptomatic pleural effusions. Aorto-esophageal branches are divided by clamping and dividing or by using an energy sealant device. This maneuver is performed under direct visualization and is facilitated by encircling the distal esophagus with a penrose drain to retract the gastroesophageal junction into the abdomen. The distal half of the esophagus can often be dissected free from the surrounding tissues under direct visualization to the level of the carina using the above techniques and varying sizes of Deaver retractors. In the setting of carcinoma, the mobility of the esophagus is assessed by grasping the tumor and a gentle rocking motion to determine whether the esophagus is fixed to the prevertebral fascia, aorta, pericardium, or trachea/bronchi. Fixation to any of these structures would preclude safe transhiatal resection. A 14-French flexible jejunostomy tube can be inserted at this point, approximately 15 cm distal to the ligament of Treitz and secured with purse-string sutures and a Witzel maneuver. We typically do not bring the jejunostomy tube through the abdominal wall until the completion of the esophagectomy.
Cervical Mobilization
In the absence of mediastinal fixation, the cervical portion of the operation proceeds next. An oblique cervical neck incision is then made parallel to the anterior border of the sternocleidomastoid muscle, typically 6–7 cm from the suprasternal notch to the level of the cricoid cartilage. The platysma muscle is divided and the sternocleidomastoid muscle is retracted laterally with the contents of the carotid sheath. The trachea is carefully retracted medially without the use of retractors to avoid injury to the recurrent laryngeal nerve in the tracheo-esophageal groove. Frequently, the middle thyroid vein and the inferior thyroid artery need to be divided for better exposure. If adequate neck extension is not possible and adequate cervical esophagus is not accessible, then a partial upper sternal split may be necessary for additional exposure to access the retrosternal esophagus [10]. The esophagus is bluntly mobilized off the prevertebral fascia posteriorly and circumferentially dissected free from the surrounding soft tissues using blunt and sharp dissection, avoiding injury to the recurrent laryngeal nerve. The cervical esophagus is elevated out of the mediastinum and encircled with a penrose drain. With superior retraction the upper thoracic esophagus is bluntly dissected free from the superior mediastinum (Fig. 3.3). The esophagus can usually be mobilized to the level of the carina with this approach avoiding injury to the membranous wall of the trachea.
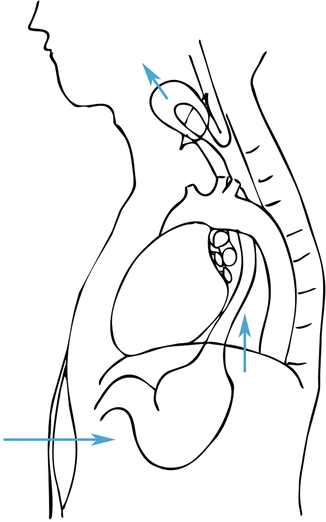

Fig. 3.3
Posterior mediastinal and posterior esophageal mobilization through the abdominal and cervical neck incisions
Detailed Mediastinal and Cervical Dissection
One hand is placed into the abdominal cavity and through the hiatus posterior to the esophagus along the right aspect of the spine to avoid aortic injury while a sponge-on-a-stick is inserted into the cervical incision posterior to the esophagus in the prevertebral plane. The posterior esophagus is bluntly dissected away from the prevertebral fascia until the hand is able to palpate the sponge stick (Fig. 3.3). It is critical to note intra-arterial blood pressure monitoring during this phase of the dissection, since hypotension is likely to occur to some degree, often necessitating removal of the hand and sponge stick from the mediastinum temporarily. At this point, a sump drain is placed into the posterior mediastinum to assess bleeding and it is left in place for the duration of the dissection. With inferior retraction of the gastroesophageal junction, the surgeon’s hand is then placed palm down on the anterior surface of the esophagus and the esophagus is dissected free from the posterior pericardium and carina. With simultaneous gentle blunt finger dissection through the cervical incision along the anterior border of the esophagus, the esophagus is completely freed from all anterior and posterior mediastinal attachments. Similar attention must be paid to systemic blood pressure during this dissection. Any remaining lateral esophageal attachments can be bluntly divided as the esophagus is elevated out of the neck incision. After this maneuver, any remaining lower esophageal attachments can be divided bluntly by inserting a hand anterior to the esophagus and placing the index and middle finger on either side of the esophagus and gently pulling these fingers inferiorly along the esophagus. Dense adhesions may need to be managed with blunt finger fracture techniques, however in most cases these can be brought down from the mediastinum for division under direct visualization. Alternatively, the adhesions can be managed by dividing the cervical esophagus and removing the esophagus and adhesions in retrograde fashion.
Esophageal and Gastric Transection and Esophagogastric Anastomosis
After circumferential mobilization of the entire intrathoracic esophagus, 8–10 cm of esophagus is delivered into the neck wound, the nasogastric tube is removed, and the esophagus divided using a linear stapler in an anterior-posterior orientation. There should be a slight oblique angle to the stapler with the tip slightly more distal than the heel. After division of the esophagus, the stomach and lower esophagus are withdrawn through the hiatus and placed on the anterior chest. A Deaver or Harrington retractor is then placed into the hiatus to inspect for mediastinal hemorrhage and entry into the pleural spaces. Bleeding vessels can be individually ligated or controlled with an energy sealant device. A laparotomy sponge is packed into the mediastinum through the hiatus at this point to help control minor bleeding.
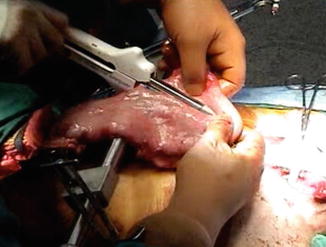
The stomach is positioned with the fundus directed cephalad. The peri-gastric fat and vessels along the lesser curve at the second vascular arcade from the cardia are divided. This marks the starting point of the gastric resection using a linear stapler. The gastric conduit should be approximately 4–5 cm wide, which typically corresponds to a gastric resection line 4–6 cm away from the gastroesophageal junction (Fig. 3.4). Following completion of the partial gastrectomy, the specimen is removed from the field and the gastric staple line is oversewn with a running 4-0 Lembert suture. The purpose of this suture is twofold: (1) to prevent staple ingrowth into surrounding structures such as the aorta or trachea and (2) to control risk of leak from the gastric staple line.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








