Fig. 1.1
Operative positioning for (a) upper midine laparotomy and (b) right fifth interspace thoracotomy
Abdominal Anatomy
Knowledge of the upper abdominal anatomy is crucial to safe and effective esophageal and gastric mobilization, performance of adequate lymphadenectomy, and creation of the gastric conduit. Relationship of the esophagus and stomach to solid organs, vascular structures, and ligamentous attachments must be considered. The esophagus enters the abdomen via the esophageal hiatus located at the level of the T8 vertebra. It is attached to the diaphragmatic crus via the phreno-esophageal ligament, which extends onto the proximal portion of the stomach. The structures considered during the dissection on the right side of the crus include the inferior vena cava, left lobe of the liver, caudate lobe of the liver, pars flaccida, duodenum, porta hepatis, and right gastric artery.
Posteriorly, the abdominal esophagus and stomach are closely related to the abdominal aorta, celiac axis, left gastric artery and vein, common hepatic and splenic arteries, pancreas, and branches of the cisterna chyle (Fig. 1.2). Inferiorly, the important structures include the right gastro-epiploic artery, greater omentum, and transverse colon and mesocolon. On the left side, the greater curvature of the stomach has attachments to the colon and spleen. After mobilization of the short gastric arteries, the tail of the pancreas and left crus come into view.
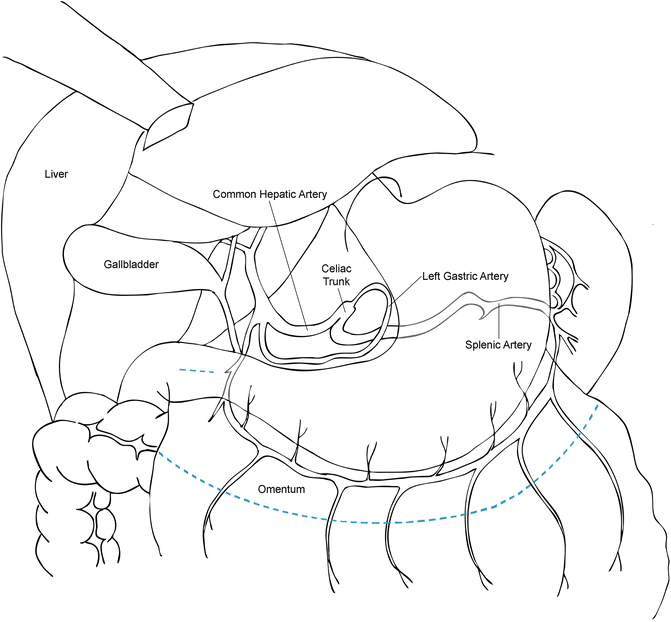

Fig. 1.2
The arterial cascade of the stomach. The blue dashed line indicates the resection line for creation of the gastric conduit and omental flap
Thoracic Anatomy
Important structures within the right thorax related to the esophagus include the thoracic aorta, azygos vein and azygos arch, thoracic duct, inferior pulmonary vein, posterior pericardium, left and right mainstem bronchi, vagus nerves along with their bronchial branches, trachea, and left recurrent laryngeal nerve. Important lymphatic basins include mediastinal level 8 and 9 lymph nodes, subcarinal level 7 lymph nodes, and paratracheal level 4 lymph nodes.
Indications for the Operation
Currently, there are several surgical techniques utilized for the resection and reconstruction of the esophagus. Ivor Lewis esophagectomy is one of those techniques; however, it does not fit all clinical scenarios in which esophageal resection may be indicated. The main decision points about the utility of the Ivor Lewis approach are the location of tumor within the esophagus and subsequently the level of anticipated esophagogastric anastomosis. Generally, the most common indication for Ivor Lewis esophagectomy is carcinoma of the esophagus located in the middle or distal esophagus and as low as the gastroesophageal junction (GEJ) and upper cardia. Other potential indications include severe esophageal stricture from gastroesophageal reflux disease or end-stage achalasia. Tumors located proximal to the level of the carina or azygos arch, or less than 25 cm from the incisors on esophagoscopy require an anastomosis in the neck and are not suitable for the Ivor Lewis approach.
Preoperative Evaluation and Imaging
Considering that esophageal carcinoma is the most frequent indication for Ivor Lewis esophagectomy, the preoperative evaluation includes adequate clinical staging along with the assessment of physiologic fitness and nutritional status of the patient. Staging of esophageal carcinoma includes esophago-gastro-duodenoscopy (EGD) with endoscopic ultrasound (EUS) to define the location of the tumor within the esophagus and the depth of esophageal wall penetration. EUS is also useful for the assessment of regional and some non-regional lymph nodes. Positron emission tomography combined with computed tomography (PET/CT) supplements staging by searching for distant metastatic disease. Ivor Lewis esophagectomy may be utilized in patients with Tis-4a, N0-3 stages.
Evaluation of the physiologic fitness and suitability for the operation is based on the surgeon’s judgment which is guided by a thorough medical history and physical examination. Adjunctive studies such as pulmonary function tests and stress echocardiogram may further help with the decision-making process. The preoperative nutritional status is very important to the overall success of the operation. Patients with esophageal adenocarcinoma usually demonstrate more robust physiognomy than patients with squamous cell carcinoma who often present in an emaciated condition. However, prolonged dysphagia due to tumor obstruction or esophagitis related to neoadjuvant radiation therapy are frequent reasons for weight loss, and must be addressed prior to definitive surgical procedure.
Perioperative Preparation
Bowel preparation or chlorhexidine shower the day before the operation are left to the discretion of the operating surgeon; they are likely more dogmatic than necessary. Clinical judgment is the best guide. While formal bowel prep is likely not necessary, preoperative constipation may manifest as significant postoperative ileus that may hinder nutrition and progress toward discharge. Our practice is to place patients on a liquid diet for 2 days prior to surgery and to use a cathartic if there is history of constipation. From an anesthesia standpoint, patients should be prepared to tolerate single lung ventilation. Epidural analgesia has become an extremely useful tool for postoperative pain control but its use must be tempered to the downside of perioperative hypotension from a sympatholytic effect. Long acting local blocks as part of a pain control cocktail are also acceptable.
Description of the Operation
Ivor Lewis esophagectomy consists of a series of operative steps and maneuvers, some which have been the source of fervent discussion and even randomized trials. Herein, we describe our modifications and approach to open Ivor Lewis esophagectomy, which can be divided into following steps: abdominal incision, mobilization of the abdominal esophagus and stomach, mobilization of the greater omental pedicle flap, dissection of the left gastric artery and D2 lymphadenectomy, pylorus draining procedure, gastric conduit creation, and feeding jejunostomy. The thoracic part of the operation can be divided into: muscle-sparing right thoracotomy incision, esophageal mobilization, thoracic duct ligation, lymphadenectomy, esophagogastric anastomosis, and omental pedicle transposition and envelope.
Abdominal Incision and Exposure
An upper midline laparotomy incision extending from just above the xiphoid process to the level of the umbilicus provides adequate exposure of the upper abdominal viscera and the omentum. In patients with central obesity, a bilateral subcostal incision with the division of the bilateral rectus abdominis muscles is an excellent alternative of exposing the upper abdomen to avoid subsequent risk of ventral incisional hernia. The blades of the Thompson retractor are placed in bilateral subcostal regions to aid the exposure of the diaphragm and the GEJ. To avoid tearing the liver capsule from excessive retractor traction, the falciform ligament may be divided to the level of the diaphragmatic attachments.
Gastric and Esophageal Mobilization
The left lobe of the liver overlying the GEJ is retracted anteriorly. Mobilization of the triangular ligament is also an option but is rarely necessary. The pars flaccida is opened, exposing the caudate lobe of the liver and the right diaphragmatic crus within the lesser sac. Then, the phreno-esophageal ligament overlying the diaphragmatic crus is incised and the crus is dissected free from the GEJ. If involved with carcinoma, a part of the crus is resected en bloc with the operative specimen to ensure negative radial margins. A penrose drain is passed around the GEJ to aid with manipulation of the esophagus and further dissection proceeds in the superior and lateral periaortic planes and along the pleural surfaces laterally reaching up into the mediastinum. This maneuver facilitates intrathoracic esophageal mobilization; however, care must be taken not to inadvertently injure or partially divide the inferior pulmonary veins on either side.
Mobilization of the Omental Pedicle
The transverse colon is lifted out of the body cavity and retracted inferiorly as the omentum is retracted superiorly. The avascular plane between the omentum and colon is opened while carefully avoiding injury to the colon, mesocolon, omentum, and greater curvature blood supply (i.e., the right gastro-epiploic vessels). The omentum is fully mobilized off the transverse colon and mesocolon after entering the lesser sac to the level of the gastroduodenal artery. Care is taken to preserve the entire course of the right gastro-epiploic artery during omental mobilization by careful palpation and/or visualization of this artery. The omental pedicle flap, based on 2–3 perforating omental arterial branches off the right gastro-epiploic artery, is created along the left side of the greater curvature (Fig. 1.2). Gastric mobilization along the greater curvature is completed with the division of the short gastric arteries staying relatively close to the stomach to avoid inadvertent splenic injury.
Abdominal D2 Lymphadenectomy and Left Gastric Artery Division
With the aid of a nasogastric tube in the stomach stretching along the greater curvature, the stomach is elevated to approach the celiac axis and left gastric artery from the lesser sac in the dissection plane created above the mesocolon. The peritoneum overlying the superior edge of the pancreas is incised to reveal the common hepatic and splenic arteries. The tissues anterior and cranial to the common hepatic and splenic arteries is included in the lymph node dissection and lymph node tissues along the left gastric artery is dissected and swept anteriorly with the specimen. The left gastric vein and artery are then divided at their origin (doubly ligated or stapled) after ensuring a good palpable pulse in both splenic and common hepatic arteries. The boundaries of dissection from right to left are the porta hepatis/vena cava to the splenic hilum, respectively; and periaortic tissues from the celiac artery inferiorly up to the extent of the mediastinal resection (Fig. 1.3). Clips should be placed on the larger lymphatics in the area of the porta hepatis to avoid chylous ascites.
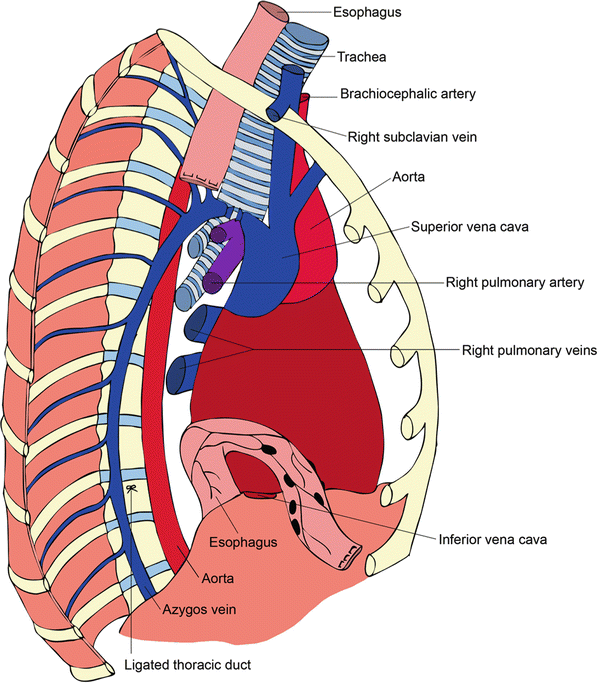

Fig. 1.3
Thoracic dissection showing ligation of the azygos arch, ligation of the thoracic duct, and resection of the esophagus with surrounding lymphatic tissues
Pylorus Draining Procedure
Controversy surrounds this portion of the procedure [3]. Some experts leave the pylorus completely intact, whereas others favor pyloromyotomy, botox injection, selective balloon dilation postoperatively, or formal pyloroplasty. Emptying of the gastric conduit through the pylorus may be related to the width of the conduit, however conclusive evidence is lacking. Our preference has been to perform either pyloromytomy or pyloroplasty.
The pylorus is identified and a 00 silk suture is placed at the superior and inferior border of the pylorus. With the cutting cautery current at 15 V the muscle tissue of the pyloric sphincter is divided and pyloromyotomy is performed; or alternatively, a Heineke-Mikulicz pyloroplasty is performed. The pyloromyotomy or pyloroplasty may be covered with a piece of omentum or previously mobilized falciform ligament and secured in place with previously placed 00 silk sutures.
Gastric Pedicle Creation
The incisura is identified at the lesser curvature of the stomach and the right gastric artery is divided above the incisura. Multiple fires of green 4.5 mm linear stapler loads aiming towards the angle of His are used to create a gastric conduit approximately 4 cm in width. In cases where the tumor extends into the stomach, care must be taken to ensure an adequate distal margin. A second staple line can be fired parallel to the first to supply the pathologist with specimen to determine the distal margin status on frozen section examination. The conduit can be completely formed in the abdomen and transferred to the chest with traction sutures, or alternatively partially formed in the abdomen with completion of the tubularization in the chest after formation of the anastomosis. Using 000 silk sutures, the omental pedicle that is to be transferred into the chest is tacked to the staple line on the proximal stomach to facilitate transposition of the gastric conduit and omentum to the chest. A feeding jejunostomy catheter is placed 30 cm from the ligament of Treitz in Witzeled fashion. Finally, the abdomen is closed.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








