Ala Abudayyeh, Abdulla Salahudeen
Onconephrology
Renal Disease in Cancer Patients
Renal disease is an important complicating feature of cancer, and also of its treatments and complications. Renal syndromes such as the syndrome of inappropriate antidiuretic hormone (SIADH) and tumor lysis syndrome (TLS) are commonly associated with cancer. However, cancer is also associated with other electrolyte abnormalities and renal syndromes (Box 68-1). The frequency of kidney complications in patients with cancer, and especially those undergoing treatment, is leading oncologists to include a nephrologist as part of a team approach for cancer therapy.1 In this chapter we review the major renal syndromes associated with cancer or its treatment.
Acute Kidney Injury
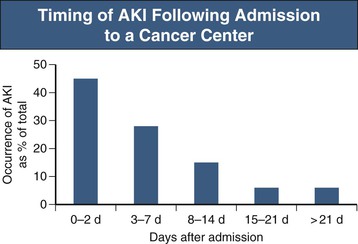
Acute kidney injury (AKI) is the most common reason why nephrologists are consulted with regard to hospitalized cancer patients and includes pre-renal, intrarenal, and post-renal AKI (see also Chapter 69). In a recent survey, the rate of AKI in patients admitted to a comprehensive cancer center was 12%, nearly threefold higher than in most noncancer settings, and AKI was also associated with poorer clinical outcomes.2,3 AKI developed in 55% of these cancer patients after they had been in the hospital for at least 48 hours (Fig. 68-1). The significant risk factors for AKI in these patients were diabetes, hyponatremia, and the use of intravenous radiocontrast, chemotherapy, and antibiotics.2 Identifying cancer patients who are likely to develop AKI in the hospital may allow kidney injury prevention (KIP) strategies to avert AKI and improve clinical outcomes.2
Pre-renal Acute Kidney Injury
Nearly one third of AKI in cancer patients is pre-renal resulting from reduced renal perfusion caused by volume depletion from chemotherapy-induced nausea, vomiting, and diarrhea. In addition, chemotherapy-associated hypoalbuminemia contributes to the reduction in effective plasma volume, adding further to the reduction in renal perfusion. Extracellular volume depletion as a result of third spacing as in malignant ascites or pleural effusion, or insensible losses from neutropenic fever can also lead to pre-renal AKI. Similarly, renal fluid loss may occur as a consequence of hypercalcemia-associated nephrogenic diabetes insipidus, which can be compounded by the renal vasoconstrictive effect of hypercalcemia (see Chapter 10).4 In patients with pre-renal dysfunction, use of nephrotoxic agents such as intravenous radiocontrast, angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), nonsteroidal anti-inflammatory drugs, calcineurin inhibitors, and various antibiotics and antiviral and antifungal agents may result in enhanced nephrotoxicity leading to intrarenal AKI.
Intrarenal Acute Kidney Injury
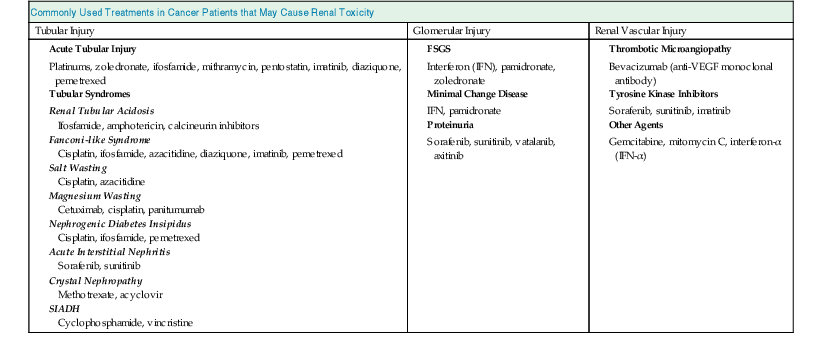
Intrarenal AKI is one of the commonest forms of renal injury in cancer patients, often preceded by pre-renal AKI. The cause is often multifactorial (see Chapter 69). Several chemotherapeutic agents as well as other medications commonly used in cancer patients can cause intrarenal AKI. AKI in this setting is referred to as toxic acute tubular necrosis (ATN), which tends to be nonoliguric and milder in severity (Table 68-1). In contrast, cancer patients with sepsis and septic shock sustain ischemic ATN, which is often oligoanuric and severe. Most of these patients with septic shock and ischemic ATN would have received chemotherapy with or without hematopoietic stem cell transplantation (HSCT) and are often neutropenic. Renal recovery is slow in ischemic ATN and often incomplete.5 Nevertheless, the presence of cancer alone should not preclude patients from being considered for dialysis. It is our practice to provide dialysis for at least 48 hours to critically ill cancer patients with advanced renal failure so that a proper evaluation can be made for the role of continuing dialysis. The majority of cancer patients in septic shock receive large volume resuscitation, blood products, and vasopressors while oligoanuric and therefore require continuous renal replacement therapy (CRRT).2,5 The occurrence of AKI remains an important obstacle for the effective use of chemotherapeutic agents. Conversely, high and repetitive doses of chemotherapy, concurrent with other nephrotoxic agents, render the patient vulnerable to AKI.
Table 68-1
Commonly used treatments in cancer patients that may cause renal toxicity.
FSGS, Focal segmental glomerulosclerosis; SIADH, syndrome of inappropriate antidiuretic hormone.
| Commonly Used Treatments in Cancer Patients that May Cause Renal Toxicity | ||
| Tubular Injury | Glomerular Injury | Renal Vascular Injury |
Acute Tubular Injury Platinums, zoledronate, ifosfamide, mithramycin, pentostatin, imatinib, diaziquone, pemetrexed | ||
Post-renal Acute Kidney Injury
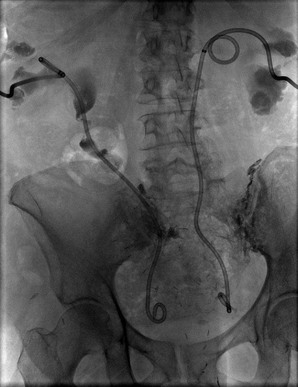
In patients with genitourinary cancers, obstruction of the urinary tract is a likely cause for acute (post-renal) AKI or chronic kidney disease (CKD). Cancers commonly associated with urinary tract obstructions are cancers of the bladder, prostate, cervix, and ovary. Among these, patients who have undergone pelvic irradiation or urogenital surgery with urinary diversion are more likely to have urinary obstruction. The obstruction seen in patients with cervical and ovarian cancers can be a result of metastatic spread involving the ureters or can be caused by ureteral or bladder compression from lymphadenopathy (Fig. 68-2). In HSCT recipients with BK infection, ureteral obstruction is not an uncommon cause of renal impairment. Although ultrasound (US) examination of kidney and bladder is the screening test for obstruction in these patients, occasionally the US findings may be negative if the obstruction is of short duration or when renal encasement by retroperitoneal cancer has occurred. In patients in whom the index of suspicion for obstruction is high, repeat US examination, nephrostogram, or both may become necessary.
Myeloma and Amyloidosis
Myeloma and amyloidosis are discussed in detail in Chapters 65 and 27, respectively. Renal involvement occurs in about 50% of patients with multiple myeloma, but the degree of renal involvement varies depending on the type of myeloma.6,7 Myeloma with light-chain overproduction is commonly associated with renal involvement, with as many as 50% of patients requiring dialysis.8 The type of light chain is also important; cast nephropathy and renal amyloidosis are common with lambda light chains, whereas severe proximal tubular injury causing Fanconi-like syndrome and light-chain deposition disease are common with kappa light chains. Treatment of myeloma with dexamethasone, thalidomide or related compounds such as lenalidomide, or proteasome inhibitors such as bortezomib, followed by myeloablative HSCT, has substantially improved the survival of myeloma patients.9 Although renal involvement of myeloma is associated with poorer clinical outcomes, kidney biopsies are not routinely performed in cancer centers. However, prompt institution of chemotherapy often leads to rapid renal recovery, occasionally obviating the need for dialysis. Reduction in serum light-chain levels with a combination of chemotherapy and plasma exchange or high-cutoff dialysis has also been shown to correlate with renal recovery, but currently there are no data to suggest that adding extracorporeal removal of light chains to myeloma therapy provides any added advantage.8 The presence of renal failure or being on dialysis is no longer a contraindication to HSCT for myeloma.10 Nevertheless, these patients require dose reduction of lenalidomide and melphalan to avoid major systemic toxicities. Unlike the initial reports that suggested that recovery of renal function after HSCT occurs in a sizable proportion of patients on dialysis because of myeloma, this seems to be the exception rather than the rule in clinical practice.11,12
Primary systemic (AL) amyloidosis in patients with light-chain disease is another infrequent manifestation of myeloma. When amyloidosis does complicate myeloma, it carries a grave prognosis. Large kidneys, gross proteinuria, and characteristic renal deposition of amyloid are hallmarks of renal amyloidosis and, based on autopsy studies, it occurs in about 5% to 10% of patients with myeloma.13 The treatment of AL amyloidosis with chemotherapy followed by HSCT has been shown to improve patient survival and reduce proteinuria.14
Anti–Vascular Endothelial Growth Factor Therapy
The discovery that vascular endothelial growth factor (VEGF) is an important mediator of tumor growth and angiogenesis led to the development of drugs targeting VEGF and VEGF receptors (VEGFRs) for cancer therapy.15 However, VEGF is also an essential growth factor to maintain glomerular endothelial health. VEGF is secreted by podocytes and traverses the basement membrane where it binds to VEGFRs on endothelial cells and maintains glomerular endothelial cell integrity and filtration barrier function (Fig. 68-3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree