11
Obesity in Aging Men and the Androgen Relationship
Case History
Mr. L.K. is a 52-year-old man who had been struggling with weight problems for the past 10 years. He reached a maximum weight of 290 pounds, and decided to be proactive and alter his lifestyle drastically. He went on a strict diet of fish, vegetables, nuts, and minimal carbohydrates and lost ∼30 pounds in 6 months. He was also prescribed Orlistat (Xenical) to help with weight loss. He wanted more dramatic results and decided to consult a plastic surgeon for help. On direct questioning, the patient confessed to easy fatigability, depressed moods, loss of libido, and erectile dysfunction. When he was seen, he weighed 260 pounds. His current medications included aspirin 325 mg, multivitamins, and protein powder. On physical examination, the patient had a blood pressure of 150/100mmHg, height 5 feet 11 inches. His cardiovascular and respiratory examination was benign. His face was bulging with excessive subcutaneous tissue, the abdominal wall was sagging, and he had multiple spider telangiectasias over the upper chest. Body fat content as measured by electrical impedance methodology was 33.6%. No masses were felt in the abdomen. Laboratory data gathered revealed total testosterone 318 ng/dL (241–827), free testosterone 10.6 pg/mL (7.2–24.0), estradiol 55pg/mL (0–54), hemoglobin (Hgb) 15.5 g/dL, total cholesterol 240mg/dL, and prostate-specific antigen (PSA) 0.7 ng/dL. He had consulted with a view toward plastic surgery as well as the possibility of androgen therapy to assist in his weight loss. He also said he wanted a physician to monitor his weight loss and to be a coach for this process.
Introducing a Relationship Between Obesity and Androgens in Men
It must be stated at the onset that andropause is a biochemical-physiological state reflecting a low level of bioavailable testosterone and may or may not be clinically symptomatic. This parallels menopause in a sense, as not all women suffer from transitory symptoms of menopause. Ultimately, however, all women suffer from the long-term effects of estrogen deprivation, such as bone, cognitive, functional, and libido loss, among others, as do andropausal men in most instances. The prevalence of obesity in aging men has been increasing in the past decade. The National Health and Nutrition Examination Survey has found that the prevalence of obesity in aging men has increased from 7.0% to 15.2% during the years 1960–1994 (Fig. 11–1).1
Men begin to be obese in middle age, and thus obesity impacts not only on mortality events but also the quality of life in later life. Obesity can be associated with an increased incidence of cardiovascular diseases, type 2 diabetes, hypertension, stroke, hyperlipidemia, osteoarthritis, and some cancers. Mortality can be also increased with obesity.2 Data on the characteristics of obesity in the aging men are somewhat limited and they vary; however, we will review the work of others and summarize our own findings. Both total and bioavailable testosterone levels decline with advancing age.3 From our research and others, it can be stated that obesity can be a strong predictor of testosterone deficiency in aging men.4 In addition to obesity, hypogonadal men may actually have reduced lean body mass and increased visceral fat. The cascade reaction may predispose aging men to an increase in diabetes and cardiovascular diseases.5 Recently, there has been a growing interest in studying the effect of testosterone replacement in aging men. However, it is prudent to have further studies especially in assessing the risk-to-benefit ratio and quality of life, so as to understand the impact of testosterone on obesity and body composition in men.
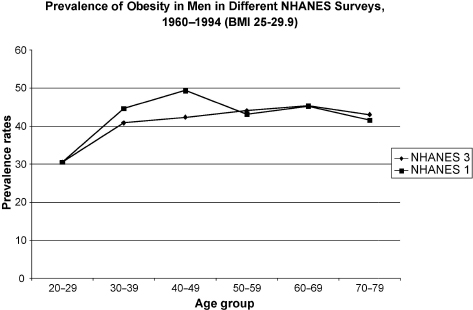
FIGURE 11-1. Obesity prevalence rates in men in the National Health and Nutrition Examination Survey (NHANES) during the years 1960 to 1994. Body mass index of 25–29.9 defines obesity. (Adapted from Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes 1998;22:39–47, with permission.)
Impact of Hypogonadism in Men
Normal aging in men is accompanied by a decline in the serum level of testosterone. Abnormalities at all levels of the hypothalamic-pituitary-testicular axis contribute to a decline in testosterone concentration in older men. Free testosterone declined by 1.2%/ year, and albumin-bound testosterone by 1.0%/year. Sex hormone-binding globulin (SHBG) increased by 1.2%/year, with the net effect that total serum testosterone declined more slowly (0.4%/year).6 Hypogonadism may not correlate directly with andropause-related symptoms. Using total testosterone as a criterion, the incidence of hypogonadism is 20% of men over 60, 30% over 70, and 50% over 80 years of age. The percentage of hypogonadism is even greater when bioavailable testosterone criteria are used.3 Despite the high prevalence rate, many men with hypogonadism remain undiagnosed and untreated. This is partly because of lack of knowledge of symptoms by both patients and providers.7,8 Besides aging, factors such as medical illness, diabetes, obesity, sleep disturbance, and medication may contribute to the decline in testosterone with advancing age. Hypogonadism in aging men can lead to symptoms such as decreased libido, erectile dysfunction, fatigue, loss of energy, change in body composition, decrease in muscle mass, increase in fat mass, muscle weakness, osteoporosis, and mood and memory changes—often classified as “andropause symptoms.”
Measuring and interpreting testosterone levels can be difficult. As such, it often confounds the results of epidemiological studies. SHBG binds testosterone, and the affinity of the binding is affected with aging process. In addition, obesity is associated with a decrease in SHBG concentration.9–11 Therefore, men with mild to moderate obesity often have low total testosterone but normal free testosterone concentration because of lower SHBG concentrations. As such, this is not real hypogonadism.11 Accordingly, androgen deficiency may be misclassified in men with low SHBG.12 In massively obese men, there is real hypogonadotrophic hypogonadism with decreased free testosterone levels, a consequence of functional alterations at the hypothalamic-pituitary-testicular axis.11 Although rare, some hypothalamic syndrome, such as Prader-Willi syndrome, can be associated with both obesity and hypogonadotropic hypogonadism. Hyperphagia-induced obesity, hypogonadism, growth hormone deficiency, and insulin and leptin resistance are all hypothalamic dysfunction in origin.13
Obesity Is a Global Public Health Concern Today
Obesity has increased steadily over the past several decades in most of the developed and developing world. The World Health Organization in 2000 proposed a classification of being overweight as body mass index (BMI) ranging from 25 to 29.9kg/m2 and obesity set at BMI 30kg/m2 or above (WHO 2000). The prevalence of obesity varies according to age, gender, race, and socioeconomic class in both developed and developing countries. The prevalence of obesity of older men and women has also increased in the last 10 years in the United States, partly because of an increasing aging population. The prevalence of obesity over 70 years of age has increased from 11.4% in 1991 to 14.6% in 1998 in United States. In addition, the prevalence of obesity increased progressively from adolescence (18–29 years: 12.1% in 1998) to age 50 to 59 years (23.8% in 1998) and then decreased progressively with age thereafter (≥70 years: 14.6% in 1998).14 Studies conducted in other countries point toward similar trends. Geographic and ethnic differences in the prevalence were large. Obesity is common in the sixth and seventh decades of life and then the rates declines. Many obese older persons were obese as middle-aged adults. After middle age, decrease in mean BMI was found in both men and women.15,16 It is interesting to note that the prevalence of obesity is low in elderly Asian men (Japan: 0.99%, Taiwan: 3.2% in 1991) but high in Middle Eastern and Western elderly men (Kuwait: 32.3%, Mexico: 15.6%, Sweden: 6.9%, United States: 15.2% in 1989).1,17 Comparative data of the decline of salivary testosterone with age seem to suggest that it is more rapid in Western as compared with Asian or South American men. In general, mean BMI among elderly women is greater for men of a similar age. Elderly men by and large have a lower prevalence of being overweight and being obese as compared with elderly women.17,18
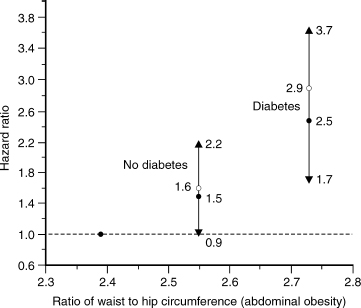
FIGURE 11-2. Relationship of abdominal obesity to risk (hazard ratio) of diabetes. A form of measure of the hazard ratio is risk of death. (Adapted from Cassano PA, Rosner B, Vokonas PS, Weiss ST. Obesity and body fat distribution in relation to the incidence of non-insulin-dependent diabetes mellitus. Am J Epidemiol 1992;136:1474–1486, with permission.)
Consequences of Obesity in Aging Men
Obesity is associated with an increased incidence of cardiovascular diseases, type 2 diabetes mellitus, hypertension, stroke, hyperlipidemia, sleep apnea, osteoarthritis, and some cancers.2 After the age of 60, body weight on average tends to decrease, but fat tends to be redistributed with advancing age toward more visceral fat.19 BMI has often been used as an indicator of overall obesity. Using BMI as a weight measure can provide an indirect measure of fatness but does not reflect fat distribution, which may reflect the risk of comorbidity independent of BMI. Moreover, BMI does not distinguish between fat mass and lean muscle mass and may sometimes underestimate fatness in older adults who have greater amount of fat at a given BMI than younger adults, due to age-related decline in muscle mass.20
Several studies have found patterns of fat distribution, particularly abdominal or central obesity, to be more closely related than BMI for increased incidence of diabetes mellitus and cardiovascular diseases.21,22
Central or abdominal obesity is represented by the ratio of waist to hip circumferences (WHR). A WHR in men greater than 1.0 indicates abdominal fat accumulation and is a risk factor for diabetes, hypertension, hyperlipidemia, and cardiovascular diseases (WHO 2000). The middle-age increase in BMI and proportion of body fat typically occurs in men after 40 years of age, and may extend to the fifth and sixth decade of life. A study on aging men confirms the correlation between abdominal obesity and the risk of diabetes (Fig. 11–2).21 Several studies have found abdominal obesity to be associated with cardiovascular disease in aging men.22,23 Greater BMI was associated with higher mortality from all causes and from cardiovascular diseases in men and women up to 75 years of age. However, the relative risk associated with greater BMI declined after 75 years of age in older men and women.16 Heiat et al24 reviewed 444 articles of overweight and obesity in elderly persons. These data do not support the BMI range of 25 to 27 as a risk factor for all-cause and cardiovascular mortality among elderly persons. Federal guideline standards for ideal weight (BMI 18.7 to < 25) may be overly restrictive as they apply to the elderly. BMI was not correlated with mortality among persons 75 years or older. However, WHR was a significant risk factor for coronary heart disease (CHD) among men 65 years or older. A longitudinal study revealed that mortality was lower in obese men compared with thin and normal older people age 70 and older.18 However, remaining free of obesity was one of the factors associated with maintenance of good health in older adults.23 The best denominators for predicting survival and sustained freedom from clinical illness and from physical and cognitive impairment in older Asian men were low blood pressure, low serum glucose, not smoking cigarettes, and not being obese.25
Summary of Study on Relationship Between Obesity, Diabetes, and Hypogonadism
As part of an ongoing quality improvement program to improve care to aging men in our geriatric clinic, we assessed and treated 71 consecutive males. Many of the patients had multiple illnesses including hypertension, diabetes, hyperlipidemia, dementia, and osteoarthritis. The routine geriatric assessments were performed including diagnoses of these patients’ age, weight, smoking, drinking, BMI, functionality scales [activities of daily living (ADL), instrumental activity of daily living (IADL), nutrition], and cognitive assessment scales [Mini–Mental Status Examination (MMSE), Clock drawing test (CDT)]. Patients had their total and free testosterone and PSA levels determined if they presented with symptomatic andropause such as fatigue, loss of libido, erectile dysfunction, mood, and memory changes. Serum total testosterone was measured by the radioimmunoassay method. Data were entered into Excel, and descriptive and comparative analyses were performed using Statistical Package for Social Scientists (SPSS).
The average age of the patients was 73 years. All 71 patients had at least one of the andropause symptoms. The mean total testosterone was 405ng/dL (range: 32–877ng/dL). Hypogonadism was defined as total testosterone <300ng/dL. Hypertension was defined as systolic blood pressure >140 mm Hg or diastolic pressure >90mmHg or current use of antihypertensive medication. Diabetes mellitus was defined as fasting blood glucose ≥126mg/dL or symptoms of diabetes and random plasma glucose ≥200mg/dL or current taking insulin or oral hypoglycemic agents. Hyperlipidemia was defined as total cholesterol ≥200mg/dL or low-density lipoprotein (LDL) cholesterol ≥130mg/dL or triglyceride ≥160mg/dL or current use of lipid-lowering agents. Thirty-one percent of this group of patients had hypogonadism, 33% had diabetes mellitus, 76% had hypertension, and 18% had hyperlipidemia. Fifty-two percent of men with low testosterone (<300ng/dL) had diabetes mellitus. Patients with diabetes were significantly more likely to be hypogonadic (p = .031). Prevalence of hypogonadism in patients with diabetes was 64%, and in those without diabetes was 38%. However, we did not find any significant association of hyperlipidemia or hypertension with hypogonadism (p = .558 and .729, respectively). On the other hand, BMI >27 was significantly related to hypogonadism (p = .026). Fig. 11–3 summarizes the results of our study, showing an inverse relationship between BMI and total testosterone.
Thirty-one percent of men who presented with andropausal symptoms had low total testosterone levels. Some studies reported that bioavailable testosterone identifies a higher proportion of men as hypogonadal than total testosterone.26 Obesity and diabetes in our group is associated with hypogonadism. The Massachusetts Male Aging Study also reported that the two strongest single predictors of testosterone deficiency were treated diabetes and obesity.27 Obese men were more likely to be hypogonadal perhaps because of increased aromatization of testosterone to estradiol in peripheral fat tissue5 and alteration of the hypothalamic-pituitary-testicular axis.11 Low testosterone may increase the activity of the hypothalamic-pituitary-adrenal axis and trigger the development of visceral obesity.28 Low testosterone also decreases the activity of lipolysis of abdominal fat.29 Some studies suggest that low testosterone plays some role in the development of insulin resistance and type 2 diabetes.30–32
The Complex Relationship Between Obesity, Body Composition, Testosterone, Growth Hormone, Insulin, Leptin, and Dehydroepiandrosterone
Aging in men involves a cascade of hormonal, biochemical, and physiological changes. As far as androgens are concerned, it is known that hypogonadal men have reduced lean body mass but an increased BMI and fat mass compared with eugonadal men of a similar age.33 Vermuellen et al34 reported in a study of 57 men aged 70 to 80 years that testosterone levels correlated negatively with percentage of body fat, abdominal fat, and plasma insulin levels. Moreover, the increase in fat mass as it occurs in aging men is in itself associated with low levels of free testosterone and growth hormone, which both normalize after weight reduction. A longitudinal male aging study reported that increased obesity may have preceded increased insulin levels or vice versa.30 Tsai et al31 established that a strong inverse relationship between the baseline level of total testosterone and accumulation of computed tomography (CT)-measured intraabdominal fat area over time in aging male. The study also showed C-peptide inversely correlated with total testosterone. This would suggest that by predisposing to an increase in visceral adiposity, low levels of testosterone might increase the risk of type 2 diabetes mellitus. Chang et al35 also showed that elderly men with type 2 diabetes had a higher WHR, BMI, skinfold thickness, and lower serum testosterone levels compared with elderly men without type 2 diabetes. A study of men born in 1913 reported that higher BMI, WHR, and lower testosterone are predictors for the development of diabetes. The diabetic state predicts strongly the incidence of myocardial infarction and stroke.36
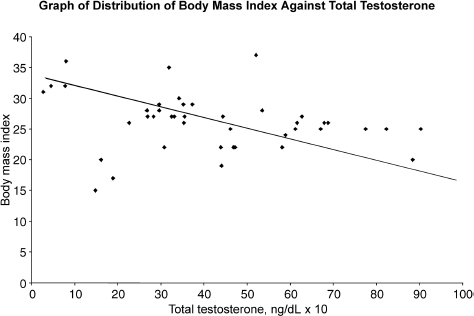
FIGURE 11-3. Relationship between body mass index and total testosterone.
Many studies have shown an association between relative hypogonadism and insulin resistance. Obesity is the most common cause of insulin resistance. Visceral obesity is associated with an increase in free fatty acid levels and decreased hepatic insulin binding and extraction.37 Intraabdominal fat may be part of the pathway through which lower testosterone is related to insulin resistance.31 Marin et al38 demonstrated that testosterone treatment could decrease visceral fat mass, insulin resistance, blood glucose, diastolic pressure, and serum cholesterol in abdominally obese men. These changes might act via the effects of testosterone on visceral fat or via direct effect on muscle insulin sensitivity. Dihydrotestosterone (DHT) treatment increases visceral fat mass and did not change insulin sensitivity and blood glucose.28 Older diabetic men with lower testosterone level are also associated with diabetic dyslipidemia.39 However, our understanding of the effect of testosterone and glucose metabolism is incomplete. In human studies, low-dose testosterone (replacement dosage) may increase insulin action, whereas high-dose testosterone (pharmacological dosage) appears to decrease insulin action.40
Leptin, the adipocyte-secreted protein product of the ob gene, has been strongly linked to obesity and is postulated to regulate weight and the adipose tissue mass by signaling satiety or hunger and energy expenditure or conservation.41 Several studies reported that leptin was correlated positively with age, BMI, serum insulin, and fat mass, and inversely with testosterone in aging men. The study of Saffele42 in healthy elderly men showed leptin level correlated positively with age, BMI, and insulin levels. Free testosterone levels were negatively correlated with age and serum leptin. Leptin level is higher in aging men with lower testosterone, and testosterone replacement normalizes elevated serum leptin levels in hypogonadal men.43–45 Cohen5 hypothesizes that the increased adipose tissue in aging men is associated with an increase in the enzyme aromatase that converts testosterone to estradiol and leads to diminishing testosterone levels. Low testosterone may contribute to the accumulation of visceral fat.5,28,46 As total body fat mass increases, hormone resistance develops for leptin and insulin. Increasing leptin fails to prevent weight gain and the hypogonadal-obesity cycle ensues, causing further visceral obesity and insulin resistance. The progressive insulin resistance can lead to diabetes and high triglyceride-low high-density lipoprotein (HDL) pattern dyslipidemia, so-called metabolic syndrome, and increase risk of cardiovascular diseases.5 It has been reported that hypotestosteronemia may be a risk factors for coronary arteriosclerosis in men.47
Testosterone is not the only androgen that declines with age. Dehydroepiandrosterone (DHEA) also declines with age. A well-known study demonstrated that dehydroepiandrosterone sulfate (DHEAS) concentration is independently and inversely related to death from any cause and death from cardiovascular disease in men over age 50.48 The Massachusetts Male Aging Study also supports the hypotheses that low DHEA and DHEAS can predict ischemic heart disease in men.49 DHEA is available without prescription in the United States. However, there are still insufficiency data to advise routine DHEA supplement in elderly men.50
Impact of Testosterone Replacement on Obesity and Body Composition
Total, free, and bioavailable testosterone levels decline progressively with age in men. Lower testosterone levels in men are associated with decreased muscle mass, increased body fat, and visceral obesity.34 Only a few studies focus on testosterone replacement on obesity and body composition in aging men. Synder et al51 analyzed 108 men over 65 years of age who were randomized to receive either testosterone patch delivering testosterone 6 mg/day or placebo; 96 completed the entire 3 years of the study. The testosterone-treated subjects experienced a significant decrease in body fat mass and an increase in lean mass. There was no significant difference of BMI between the two groups (Fig. 11–4).
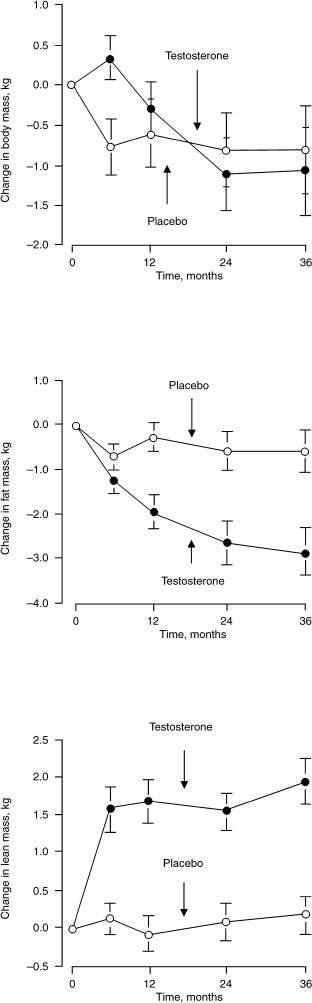
FIGURE 11-4. Effect of testosterone on body fat and lean mass in men over 65 years of age. (Adapted from Synder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 1999; 84:2647–2653, with permission.)
Sih et al52 reported that testosterone replacement with 200 mg testosterone cypionate biweekly for 12 months improved muscle strength and lowered leptin levels in older hypogonadal men over 65 years of age. Fig. 11–5 shows the effects of testosterone on leptin.
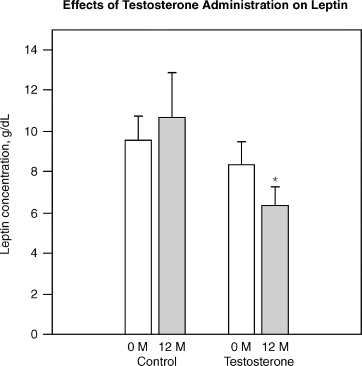
FIGURE 11-5. Effect of testosterone on leptin, suggesting testosterone can decrease leptin resistance. Leptin is often elevated in obesity and the rise is a measure of leptin resistance. Testosterone has been shown in clinical trials to decrease leptin suggesting that it may control obesity. (Adapted from Sih R, Morley JE, Kaiser FE, Perry HM, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol 1997;82:1661–1667, with permission.)
Testosterone did not alter body fat or BMI in this study.52 A retrospective analysis of testosterone replacement with 200 mg testosterone enanthate or cypionate every 2 weeks in older hypogonadal men showed no significant differences in body weight and BMI in two groups during 2 years follow-up.53 Morley et al52 treated eight hypogonadal men, aged 79 to 89 years, with testosterone enanthate 200mg every 2 weeks for 3 months, and observed no changes in fat mass or body weight, although they did observe an increase in grip strength. Tenover54 treated 70 hypogonadal men, aged 65 to 83 years, with testosterone enanthate 150 mg twice a week for up to 3 years and observed an increase in lean body mass as well as decrease in body fat. Wang et al55 treated 227 hypogonadal men with transdermal testosterone gel (50–100 mg/day) for 180 days and observed an increase in lean mass and a decrease in fat mass. Taken together, these data suggest that variation in responsiveness of fat to testosterone replacement depends on the duration of therapy. Other factors such as pretreatment body composition, the method of body composition assessment, and the age of the subjects might also affect the responsiveness of body fat to testosterone administration. Testosterone replacement is absolutely contraindicated in men with prostate cancer or breast cancer. Thorough urological evaluation should be performed before testosterone replacement and frequent monitoring of PSA in older men during receiving testosterone replacement is indicated. The other potential risks of testosterone therapy in men include leg swelling, gynecomastia, sleep apnea, and polycythemia. Liver toxicity has not been a significant issue with injectable esters, transdermal patches, or gel.
Discussion of the Case History
Mr. L.K. did receive androgen therapy, in the form of compounded testosterone 5 mg with 100 mg of zinc in a topical form. The zinc was added to act as a potential aromatase inhibitor. Oral zinc is not tolerated well in larger doses as it can cause diarrhea and vomiting, and for that reason it was applied topically. The pharmacokinetics and pharmacodynamics of absorbed zinc has been studied and documented. Testosterone was prescribed as his levels were low normal, and he suffered the symptoms. The androgens given boosted his energy level, which allowed him to go the gymnasium 6 days a week. He did a combination of weight training and aerobic exercise in 30 minutes sessions each. His libido returned, although a phosphodiesterase inhibitor had to be prescribed to aid his erectile function. He did undergo a series of plastic surgeries over a 6-month period, including liposuction of his chin and abdomen. He subsequently underwent an abdoplasty as well. At a follow-up visit, he mentioned that he was very happy with himself, and that he is now religiously following his diet and exercise regimen. On examination, he had surgical scars from his surgery, body fat content is down to 23.8%, and blood pressure is 132/88 mm Hg. His laboratory data include total testosterone 560 ng/dL, estradiol 40 pg/dL, and total cholesterol 190 mg/dL. Overall, the patient looks different and he reports he feels different, and he believes he is living life again. This is an example of a dramatic and drastic attempt of an older man wanting to lose weight for an improved quality of life.
Conclusion and Key Points
In most studies mean body weight or BMI increases with age up to about age 60 to 70 and then declines with age. Some studies actually suggest a protective effect of overweight in the oldest age groups. Visceral obesity may be a better indicator of risk of diabetes mellitus and related cardiovascular disorders than BMI in aging men.
Obesity is an issue that is increasingly affecting aging men not only in developed countries but also developing ones. Excess wealth and poor education lead to bad eating habits. With aging, there is a decline in androgens as well. There are implications for the health of men as a result of hypogonadism. Overall, there seems to be an inverse relationship between BMI and testosterone levels. Severe obesity seems to suppress the production of testosterone. It has been hypothesized that there is increased aromatization of testosterone to estradiol and alteration of hypothalamic-pituitary-adrenal axis in obese older men. Some hormones can affect obesity in aging, including leptin, insulin, DHEA, and growth hormone. The relationship of obesity to these hormones in the aging men was reviewed. Testosterone replacement can alter body composition whereby fat may be exchanged for muscle. Further studies in this field are recommended to evaluate long-term benefits and risks.
• Obesity is reaching epidemic portions not only in developed nations but also in the developing world.
• Overall, there seems to be an inverse relationship between BMI and testosterone.
• Obesity not only affects cardiovascular and cerebrovascular morbidity, but also may impact androgens and hence quality of life.
• Low testosterone is associated with insulin resistance and high leptin.
• There is a possibility of a reduction in obesity and the associated metabolic syndrome in some cases with physiological doses of testosterone replacement.
REFERENCES
1. Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Over-weigh and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord 1998;22:39–47
2. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA 1999;282:1523–1529
3. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 2001;86:724–731
4. Tan RS. The Andropause Mystery. Houston: AMRED Publishing, 2001
5. Cohen PG. Aromatase, adiposity, aging and disease: the hypogonadal-metabolic-atherogenic-disease and aging connection. Med Hypotheses 2001;56:702–708
6. Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab 1991;73:1016–1025
7. Tan RS, Philip PS. Perceptions of and risk factors for andropause. Arch Androl 1999;43:97–103
8. Bhasin S, Buckwalter JG. Testosterone supplementation in older men: a rational idea whose time has not yet come. J Androl 2001;22:718–731
9. Haffner SM, Valdz RA, Stern MP, Katz MS. Obesity, body fat distribution and sex hormones in men. Int J Obes Relat Metab Disord 1993;17:643–649
10. Ukkola O, Gagnon J, Rankinen T, et al. Age, body mass index, race and other determinants of steroid hormone variability: the HERITAGE Family Study. Eur J Endocrinol 2001;145:1–9
11. Vermeulen A. Decreased androgen levels and obesity in men. Ann Med 1996;28:13–15
12. Winters SJ, Kelly DE, Goodpaster B. The analog free testosterone assay: are the results in men clinically useful? Clin Chem 1998;44:2178–2182
13. Nagai T, Mori M. Prader-Willi syndrome, diabetes mellitus and hypogonadism. Biomed Pharmacother 1999;53:452–454
14. Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP The spread of the obesity epidemic in the United States, 1991–1998. JAMA 1999;282:1519–1522
15. Launer LJ, Harris T. Weight, height and body mass index distribution in geographically and ethically diverse samples of older persons. Age Ageing 1996;25:300–306
16. Steven J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body mass index and mortality. N Engl J Med 1998;338:1–7
17. Chiu HC, Chang HY, Mau LW, Lee TK, Liu HW. Height, weight and body mass index of elderly persons in Taiwan. J Gerontol A Biol Sci Med Sci 2000;55:M684–M690
18. Lerman-Garber I, Villa AR, Martinez CL, et al. The prevalence of obesity and its determinants in urban and rural aging Mexican populations. Obes Res 1999;7:402–406
19. Siedell JC, Visscher TL. Body weight and weight change and their health implications for the elderly. Eur J Clin Nutr 2000;54:S33–S39
20. Teh BH, Pan WH, Chen CJ. The relocation of body fat toward the abdomen persists to very old age, while body mass index declines after middle age in Chinese. Int J Obes Relat Metab Disord 1996;20:683–687
21. Cassano PA, Rosner B, Vokonas PS, Weiss ST. Obesity and body fat distribution in relation to the incidence of non-insulin-dependent diabetes mellitus. Am J Epidemiol 1992; 136:1474–1486
22. Grinker JA, Tucker KL, Vokonas PS, Rush D. Changes in patterns of fatness in adult men in relation to serum indices of cardiovascular risk: the normative aging study. Int J Obes Relat Metab Disord 2000;24:1369–1378
23. Burke GL, Arnold AM, Bild DE, et al. Factors associated with healthy aging: the cardiovascular health study. J Am Geriatr Soc 2001;49:254–262
24. Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med 2001;161:1194–1203
25. Reed DM, Foley DJ, White LR, Heimovitz H, Burchfiel CM, Masaki K. Predictors of healthy aging in men with high life expectancy. Am J Public Health 1998;88:1463–1468
26. Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000;49:1239–1242
27. Smith KW, Feldmen HA, McKinlay JB. Construction and field validation of a self-administered screener for testosterone deficiency (hypogonadism) in aging men. Clin Endocrinol (Oxford) 2000;53:709–711
28. Marin P, Arver S. Androgen and abdominal obesity. Baillieres Clin Endocrinol Metab 1998;12:441–451
29. Marin P, Oden B, Bjorntorp P. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effect of androgens. J Clin Endocrinol Metab 1995;80:239–243
30. Lazarus R, Sparrow D, Weiss S. Temporal relation between obesity and insulin: longitudinal data from the Normative Aging Study. Am J Epidemiol 1998;147:173–179
31. Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord 2000;24:485–491
32. Goodman-Gruen D, Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care 2000;23:912–918
33. Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab 1996;81:4358–4365
34. Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Invest 1999;22:110–116
35. Chang TC, Tung CC, Hsiao YL. Hormonal changes in elderly men with non-insulin-dependent diabetes mellitus and the hormonal relationship to abdominal adiposity. Gerontology 1994; 40:260–267
36. Tibblin G, Adlerberth A, Lindstedt G, Bjorntorp P. The pituitary-gonadal axis and health in elderly men. A study of men born in 1913. Diabetes 1996;45:1605–1609
37. Lemieux S. Contribution of visceral obesity to insulin resistance syndrome. Can J Appl Physiol 2001;26:273–290
38. Marin P, Holmang S, Jonsson L, et al. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord 1992;16:991–997
39. Barrett-Connor E. Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med 1992;117:807–811
40. Rizza R. A. Androgen effect on insulin action and glucose metabolism. Mayo Clin Proc 2000;75(suppl):S61–S64
41. Bray GA, York DA. Clinical review 90: leptin and clinical medicine: a new piece in the puzzle of obesity. J Clin Endocrinol Metab 1997;82:2771–2776
42. Saffele JKV, Goemaere S, Bacquer DD. Serum leptin levels in healthy ageing men: are decreased serum testosterone and increased adiposity in elderly men the consequence of leptin deficiency? Clin Endocrinol (Oxford) 1999;55:81–88
43. Perry HM III, Miller DK, Morley PP. Testosterone and leptin in older African-American men: relationship to age, strength, function, and season. Metabolism 2000;49:1085–1091
44. Jockenhovel F, Blum WF, Vogel E, et al. Testosterone substitution normalizes elevated serum leptin levels in hypogonadal men. J Clin Endocrinol Metab 1997;82:2510–2513
45. Luukkaa V, Pesonen U, Huhtaniemi I, et al. Inverse correlation between serum testosterone and leptin in men. J Clin Endocrinol Metab 1998;83:3243–3246
46. Marin P. Testosterone and regional fat distribution. Obes Res 1995;(Suppl 4):609S–612S
47. Phillips GB, Pinkernell BH, Jing TY. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler Thromb 1994;14:701–706
48. Barrett-Connor E, Khaw KT, Yen SS. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med 1986;315:1519–1524
49. Feldman HA, Johannes CB, Araujo AB, Mohr BA, Longcope C, McKinlay JB. Low dehydroepiandrosterone and ischemic heart disease in middle-aged men: perspective results from the Massachusetts Male Aging Study Am J Epidemiol 2001;153: 79–89
50. Porsova-Dutoit I, Sulcova J, Starka L. Do DHEA/DHEAS play a protective role in coronary heart disease? Physiol Res 2000;49(suppl 1):S43–S56
51. Synder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 1999;84: 2647–2653
52. Sih R, Morley JE, Kaiser FE, Perry HM, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab 1997;82:1661–1667
53. Hajjar RR, Kaiser FE, Morle JE. Outcomes of long-term testosterone replacement in older hypogonadal males: a retrospective analysis. J Clin Endocrinol Metab 1997;82: 3793–3796
54. Tenover JS. Effects of testosterone supplementation in aging male. In: 1st World Congress, “The Aging Male” Geneva: 1997
55. Wang C, Swerdloff RS, Iranmanesh A, et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. Testosterone Gel Study Group. J Clin Metab 2000;85: 2839–2853
< div class='tao-gold-member'>









