Chapter 12 Nonvariceal Upper Gastrointestinal Bleeding
Introduction
Upper gastrointestinal (GI) bleeding is a common GI emergency. Frequency of occurrence is 40 to 150 cases per 100,000 in the Western population, and it accounts for a total expenditure of $2.5 billion annually in the United States.1,2 Significant advances have been made not only in decreasing the overall incidence, but also in treatment (medical treatment and endoscopic hemostasis), prevention of complications, and recurrence of bleeding.3 These advances have resulted in an overall decrease in the rate of hospitalizations and mortality associated with upper GI hemorrhage in the Western world.4 However, more recent data suggest that although hospital admission rates may have decreased, patients being admitted are older and have more comorbid illnesses. These patients are known to carry a worse prognosis.3 Acute nonvariceal upper GI bleeding is still associated with significant morbidity and mortality despite significant development in the understanding of the pathophysiology of the disease and the armamentarium available to the endoscopist for endoscopic therapy to treat these lesions. This chapter discusses the approach, assessment, and management strategies in patients presenting with nonvariceal upper GI bleeding.
Clinical Presentation
The presentation of GI bleeding depends on the volume and site of bleeding. Hematemesis is vomiting of blood and is the most common presentation of an upper GI bleed. The source is almost always proximal to the ligament of Treitz. Blood may be bright red, indicating a recent bleed, or resemble “coffee grounds,” representing older blood reduced by acid in the stomach. Melena is black, tarry, and sticky stool with a specific foul odor caused by degradation of blood in the intestines and colon. This presentation most commonly indicates an upper GI source; however, blood from the right colon may also manifest as melena. A massive upper GI bleed can manifest as hematochezia (bright red blood per rectum) in 15% of cases and carries a worse prognosis.5
Initial Evaluation
The initial assessment of a patient suspected to have an acute GI bleed must focus on hemodynamic stability so that resuscitative measures can be started without delay. Good clinical assessment determines the fluid requirement for hemodynamic stabilization.6 A focused history and physical examination provides vital information on the severity of bleeding and other confounding medical problems (e.g., coronary disease, chronic obstructive pulmonary disease, malignancy) that may affect medical management and therapeutic intervention. The initial evaluation should focus on vital signs and orthostatic changes because postural hypotension represents a significant volume loss (>15%) and is predictive of a poor outcome (Table 12.1).7,8
Nasogastric Aspirate
Nasogastric tubes can be helpful in the localization of bleeding because a positive nasogastric aspirate (coffee ground or bright red blood) confirms the source to be from the upper GI tract. However, the localization of bleeding can be determined by a careful history and physical examination. A reliable history of hematemesis confirms the source to be proximal to the ligament of Treitz. Use of a nasogastric tube to stratify patients with high-risk lesions is controversial.9 In the national survey of the American Society of Gastrointestinal Endoscopy (ASGE), about 16% of patients with clear nasogastric aspirates were found to have active bleeding at the time of endoscopy.10 The bleeding sources in these patients also included esophagitis (10.7%) and varices (5.1%).
Aljebreen and colleagues11 performed a retrospective analysis on 1869 patients with upper GI bleeding. A bloody nasogastric aspirate was significantly associated with high-risk lesions (active bleeding, nonbleeding visible vessel, adherent clot). A bloody aspirate had a specificity of 75.8% and negative predictive value of 77.9% for high-risk lesions. Although nasogastric lavage provides no information about the etiology of bleeding, it can be valuable in localizing the source in a hemodynamically unstable patient because it is a quick and easy test that is performed at bedside. A negative or bilious aspirate does not rule out upper GI bleeding. Nasogastric suction often is not needed with the availability of large-channel therapeutic endoscopes, with forward water jets built into the endoscope and the ability to suction directly from the endoscopic channel port, bypassing the internal suction channeling that passes through the handle of the endoscope and through the umbilicord, which can contain a stepdown in channel size.
Laboratory Data
Laboratory tests appropriate at initial presentation include hemoglobin level, hematocrit, platelet count, prothrombin time, and partial thromboplastin time. Initial hemoglobin level may not depict the degree of bleeding, and initial decision making must be based on clinical grounds. An increased blood urea nitrogen (BUN)-to-creatinine ratio (>36) has been suggestive of an upper GI source of bleeding with a sensitivity of 90% and specificity of 27%.12 BUN levels can weakly predict severity of bleeding but are not helpful in predicting high-risk lesions.13 BUN values can be helpful in the diagnosis; however, the clinical picture is often complicated by other medical illnesses (e.g., renal insufficiency, congestive heart failure) and polypharmacy. Other important laboratory data include liver function tests and cardiac enzyme analysis.
Risk Stratification
Most GI bleeds stop spontaneously without any recurrence. Approximately 20% of bleeds can continue or recur leading to patient morbidity and mortality.14 Numerous clinical factors have been identified that predict a high rate of recurrent bleeding or poor outcome. Old age (>65 years), shock, comorbid illnesses, low hemoglobin on evaluation, melena, multiple transfusions (more than five), hematochezia, fresh blood emesis or nasogastric aspirate, and need for emergency surgery are clinical predictors of increased risk of rebleeding.15,16 A history of alcoholism, a history of cancer, and an Acute Physiology, Age, and Chronic Health Evaluation (APACHE) II score of 11 or greater have also been associated with poor outcomes.17,18
Chiu and colleagues19 prospectively studied 3220 patients with bleeding peptic ulcers from 1993 to 2003 and identified risk factors for mortality. Among patients with bleeding peptic ulcers after endoscopic hemostasis, advanced age, multiple comorbidities, hypovolemic shock, in-hospital bleeding, rebleeding, and need for surgery were significant factors predicting in-hospital mortality (Table 12.2). Based on these individual clinical criteria and endoscopic findings, numerous scoring systems have been formulated to try to stratify high-risk patients for appropriate intervention.
Table 12.2 Statistically Significant Predictors of Persistent or Recurrent Bleeding
| Risk Factor | Odds Ratio for Increased Risk |
|---|---|
| Age | |
| >65 yr | 1.3 |
| >70 yr | 2.3 |
| Shock (systolic blood pressure <100 mm Hg) | 1.2–3.65 |
| Comorbid illness | 1.6–7.63 |
| Transfusion requirements | NA |
| Initial hemoglobin <10 g/dL | 0.8–2.99 |
| Coagulopathy (prolonged prothrombin time) | 1.96 (1.46–2.64) |
| Melena | 1.6 |
| Blood in nasogastric tube or stomach | 1.1–11.5 |
| Hematemesis | 1.2–5.7 |
| Continued bleeding | 3.14 (2.4–4.12) |
| Need for emergency surgery | NA |
NA, Not available.
Modified from Barkun A, Bardou M, Marshall JK; Nonvariceal Upper GI Bleeding Consensus Conference Group: Consensus recommendations for managing patients with non-variceal upper gastrointestinal bleeding. Ann Intern Med 139:843–859, 2003.
Blatchford and associates20 devised a scoring system based on admission hemoglobin, BUN, pulse, systolic blood pressure, presentation with syncope and melena, and evidence of hepatic disease and cardiac failure. Their criteria could identify 20% of patients with very low risk of needing treatment to control bleeding, who could be offered outpatient treatment. The Blatchford scoring system has been validated by other investigators.21 In 1974, Forrest and colleagues23 first classified stigmata of active or recent bleeding based on visualization at the time of endoscopy (Table 12.3). Visible vessel is the term used for an elevated area within the ulcer base. It is thought to be a coagulum over a small-caliber arterial vessel in the ulcer base.24 The color of the lesion can also be predictive of rebleeding: Nonpigmented lesions (white-pale) have a higher risk (71%) than pigmented lesions (38%).25 The frequency with which a visible vessel can be found also depends on the timing of endoscopy and how aggressively the clot is washed to expose the ulcer base. Chung and coworkers26 reported disappearance of visible vessels in 62 patients who underwent endoscopy for 3 consecutive days.
Table 12.3 Modified Forrest Criteria
| Forrest Class | Type of Lesion |
|---|---|
| IA | Arterial spurting |
| IB | Active oozing |
| IIA | Ulcer with nonbleeding visible vessel |
| IIB | Ulcer with adherent clot on surface |
| IIC | Ulcer with red or dark blue flat spot |
| III | Ulcer with clean base |
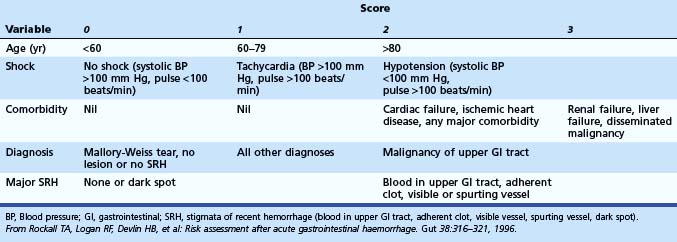
Rockall and colleagues27 developed a scoring system involving clinical and endoscopic criteria. The main aim is to predict risk of rebleeding and mortality. An initial calculation is based on clinical parameters, and further categorization of the bleeding lesion and the stigmata of hemorrhage at the time of endoscopy enables the complete score to be calculated. A total score of less than 3 was associated with excellent prognosis, whereas a score greater than 8 carried a high mortality. For cases with a score less than 3, rebleeding occurred in less than 5%, and mortality was 0%. Multiple studies have been performed to validate the Rockall scoring system.28,29 It has been validated in other studies to be predictive and cost-effective (Table 12.4).30
Table 12.4 Rockall Scoring System for Risk of Rebleeding and Death after Admission to the Hospital for Acute Gastrointestinal Bleeding
Hay and coworkers31 derived a scoring system by using hemodynamics, time from bleeding, comorbidity, and endoscopy findings. Use of the practice guidelines reduced hospital stay in low-risk patients from 4.6 to 2.9 days (P < .001). Saeed and associates32 proposed another scoring system based on endoscopy findings. In a retrospective analysis, Chuan and colleagues compared the Blatchford score with the Rockall score in 354 patients. The Blatchford score identified 92.1% of patients, whereas the Rockall score identified 70.1%. Blatchford scoring based on clinical and laboratory parameters was concluded to have a higher sensitivity to identify high-risk patients (P < .0001).33,34
Kim and associates35 conducted a prospective study to compare the clinical utility of five scoring systems for the prediction of rebleeding and death in patients with nonvariceal upper GI bleeding. Using five scoring systems (Forrest classification, Rockall scoring system, Cedars-Sinai Medical Center Predict Index, Blatchford scoring system, and Baylor College scoring system), 239 consecutive patients were investigated. All patients underwent endoscopy for nonvariceal bleed. There were 35 patients (14.6%) who experienced rebleeding and 20 patients (8.4%) who died. Forrest classification was superior to the others in predicting rebleeding and death. The Cedars-Sinai Medical Center Predict Index and the Rockall scoring system showed high positive predictive values for predicting rebleeding (Cedars-Sinai Medical Center Predict Index) and death (Rockall scoring system). Forrest classification was thought to be the most useful scoring system for the prediction of rebleeding and death in patients with nonvariceal upper GI bleeding. The development of clinical scoring systems is very encouraging because these can be used by emergency department physicians, general practitioners, and junior house staff to triage patients effectively. These scoring systems have been validated in large cohorts in diverse populations and have been shown to be effective in risk stratification and cost-effectiveness.
Timing of Endoscopy
Endoscopy primarily is used to determine the cause of bleeding, prognosticate, and administer appropriate endoscopic therapy to control bleeding and prevent rebleeding.36,37 The timing of endoscopy has been a subject of debate, especially in patients who are clinically stable with no evidence of further bleeding. It is now evident that early endoscopy provides essential data affecting patient triage.
Lee and associates38 showed that early “endoscopy triage” leads to early discharge of patients with low risk of rebleeding, without increasing morbidity and mortality. Median cost savings were $2068. Cipolletta and coworkers39 used endoscopic and clinical criteria to identify patients at low risk for recurrent bleeding. Patients were randomly assigned to outpatient care versus hospital admission. No patients underwent surgery or died. Rates of recurrent bleeding were comparable (2.1% and 2.2%) in both groups. Median costs were $340 for outpatients and $3940 for inpatients. Based on multiple studies confirming the beneficial effects of endoscopic therapy, urgent endoscopy has been recommended by the National Institutes of Health40 and ASGE41 for patients who have active bleeding or who are at high risk for rebleeding. The definition of urgent endoscopy ranges in various studies from 2 to 24 hours after presentation to the hospital. It has been determined that 76% to 78% of patients with acute GI bleeding undergo endoscopy within the first 24 hours.42,43 Even if discharge is not always the best option, triage results might help choose the correct level of care within the hospital.
Preparation and Place for Endoscopy
Endoscopy is best performed in a fully equipped endoscopy suite where staff members are trained to take care of the patient and in the use and maintenance of endoscopes and their accessories (Fig. 12.1). A general recommendation is that patients with mild to moderate bleeding can have endoscopy the next day in the endoscopy unit. Patients whose presentation suggests a major or severe bleeding event (e.g., hemodynamic compromise) can undergo endoscopy at the bedside in the intensive care unit after initial resuscitation. Endotracheal intubation to protect the airway and prevent aspiration should be considered in high-risk patients who are experiencing active hematemesis, marked agitation, lethargy, and persistent shock. In the United States, there is increasing use of propofol for routine and even emergency endoscopy; use of propofol not administered by an anesthetist has also been studied but is not widely employed in current practice.44
In patients with active or recent hemorrhage, residual blood in the stomach, especially in the fundus, can limit the quality of the examination, and residual clots must be removed or flushed away. Poor visualization at the time of endoscopy has been associated with worse outcomes.45 Multiple methods have been proposed to overcome this problem. Vigorous gastric lavage using a large-bore nasogastric tube at the time of endoscopy and instillation of 3% hydrogen peroxide46 to dissolve small clots have been proposed. Use of an endoscope with a large device channel may offer a convenient and effective method to remove clots and blood from the stomach.47,48 Connecting a suction line directly to the endoscope device channel, bypassing the internal system, provides rapid and impressive clearing of blood and clot. Randomized controlled trials have looked into using erythromycin as a promotility agent to improve quality and yield of endoscopy.49–52 Patients with acute GI bleeding were randomly assigned to receive erythromycin (3 mg/kg intravenously) and were compared with a no treatment arm. The treatment group (erythromycin 3 mg/kg intravenously 20 to 60 minutes or 120 minutes before endoscopy) had an improved quality of endoscopic examination and reduced need for second-look endoscopy, and erythromycin was deemed a cost-effective strategy.
Etiology of Nonvariceal Upper Gastrointestinal Bleeding
Causes of nonvariceal upper GI bleeding are presented in Table 12.5.
Table 12.5 American Society of Gastrointestinal Endoscopy Bleeding Survey: Endoscopic Diagnosis for Upper Gastrointestinal Bleeding in 2225 Patients
| Diagnosis | Frequency (%) |
|---|---|
| Duodenal ulcer | 24.3 |
| Gastric erosions | 23.4 |
| Gastric ulcer | 21.3 |
| Varices | 10.3 |
| Mallory-Weiss tear | 7.2 |
| Esophagitis | 6.3 |
| Erosive duodenitis | 5.8 |
| Neoplasm | 2.9 |
| Stomal ulcer | 1.8 |
| Esophageal ulcer | 1.7 |
| Miscellaneous | 6.8 |
Gastric and Duodenal Ulcers
Gastroduodenal ulcer disease is the most common cause of acute GI bleeding in the Western world despite an overall decline in the disease incidence in the last 3 decades.53–56 Ulcer disease is responsible for 50% of patients presenting with upper GI bleeding.57 With better understanding of the risk factors and better treatment protocols, overall prevalence and hospital admissions for peptic ulcer disease have decreased. The hospitalization rate for ulcer-related GI bleeding has not changed significantly, however.56 More elderly patients (>65 years old) are being hospitalized with bleeding ulcers.54,58–60 Mortality associated with peptic ulcer disease is still about 14% and is known to increase progressively with age.14,61 Previously, all peptic ulcers were considered idiopathic; in the 1980s and 1990s, most ulcer disease was attributed to Helicobacter pylori and nonsteroidal antiinflammatory drugs (NSAIDs). H. pylori was associated with 90% of duodenal ulcers and 70% of gastric ulcers.62 With awareness and aggressive therapy, there has been a decline in the prevalence of H. pylori in the Western world. The epidemiology of peptic ulcer disease has changed with a much higher percentage of H. pylori–negative ulcer disease now being reported.63,64
Mamdani and colleagues65 reported an increase in admission rate from upper GI bleeding in patients older than 65 years with greater use of cyclooxygenase-2 (COX-2) and NSAIDs. H. pylori infection and NSAID use both increase risk of peptic ulcer disease and upper GI bleeding.14,66–68 In a meta-analysis by Huang and colleagues,66 NSAID use was significantly greater in patients with peptic ulcer disease than in matched controls. H. pylori infection marginally increased the risk of ulcer bleeding. Risk of bleeding was much greater when both risk factors coexisted, having an additive effect. COX-2 inhibitors increase the risk modestly—less than NSAID use but higher than no use at all.69 Cardiovascular risks associated with some COX-2 inhibitors must also be taken into consideration. Patients seen with GI bleeding with ulcers with high-risk features (active bleeding, nonbleeding visible vessel) commonly have continued or recurrent bleeding, and up to 35% require urgent surgery.70 Over the last 15 to 20 years, endoscopic therapy has been shown to benefit this population of patients. Endoscopic therapy has been associated with reduction in the rates of rebleeding, blood transfusion, length of hospital stay, need for other therapeutic interventions, costs, and mortality.71–73
Indications for Endoscopic Therapy for Gastroduodenal Ulcers
After initial resuscitation of patients with severe upper GI bleeding and initiation of medical therapy for patients with suspected ulcer hemorrhage, urgent endoscopy is integral to the initial care, permitting effective triage of patients into low-risk and high-risk categories. Endoscopic treatment directed at major stigmata for ulcer hemorrhage should be intentionally planned because it has shown to improve outcome. A multicenter U.S. trial of 4090 patients hospitalized for nonvariceal GI bleeding showed 10.3% had active bleeding (arterial or oozing), 12.2% had nonbleeding visible vessel, 8.3% had adherent clot, 9.9% had flat spot, and 58.4% had an ulcer with clean base.74 Endoscopic treatment is not recommended for patients with low-risk endoscopic stigmata, including an ulcer with a clean base or a dark nonprotuberant pigmented spot in the ulcer base. Patients who have active bleeding, spurting or oozing from the ulcer, or a nonbleeding visible vessel in the ulcer base should receive endoscopic therapy.75,76 In a meta-analysis by Bardou and colleagues,77 endoscopic treatment was associated with statistically significant absolute decrease in rates of rebleeding, surgery, and mortality.
Nonbleeding Adherent Clot
Endoscopic therapy for ulcers with an adherent clot is also recommended. A high rate of rebleeding has been documented in these patients (33% to 40%) caused by an underlying visible vessel. Earlier studies did not show any benefit of endoscopic monotherapy in ulcers with adherent clots.78,79 Severe bleeding may be induced from removal of the clot.76 This observation has often been a deterrent to intervention and has led to confusion regarding whether or not to intervene. Later studies have shown combination therapy to be superior to medical therapy.
In a multicenter study organized by the Mayo Clinic GI Bleeding Team, Bleau and colleagues80 randomly assigned patients with adherent clots to receive medical therapy or endoscopic therapy. In the treatment arm, 1 : 10,000 epinephrine was injected in four quadrants around the ulcer before removing the overlying clot aggressively (cold snare, suction, manipulation with biopsy forceps and tip of the endoscope). Underlying stigmata were treated with heater probe coagulation. Rates of rebleeding were 34.3% in the medical treatment arm and 4.8% in the endoscopic treatment arm. Jensen and coworkers81 randomly assigned 32 patients with severe bleeding and adherent clots to combination endoscopic therapy or medical management. In their study, after epinephrine injection in four quadrants, cold guillotining was used to shave off the clot to 3 to 4 mm above the ulcer base. The residual clot was treated with coaptive coagulation. Rebleeding rate in the medical treatment group was 35.3% and in the endoscopic therapy group was 0%.
A meta-analysis by Kahi and associates82 of six studies comprising 240 patients showed significant reduction in rebleeding rates (relative risk 0.35, 95% confidence interval [CI] 0.14 to 0.83) in patients who received endoscopic treatment versus medical treatment alone (8.2% vs. 24.7%). Other outcomes, such as length of hospital stay, need for surgery, transfusion requirements, and surgery, were not significant. The underlying mechanism of rebleeding in the case of an adherent clot is thought to be stigmata underlying the clot, which are the major determinants of rebleeding. Evaluation of the ulcer base is vital. Injection of 1 : 10,000 epinephrine seems to reduce the risk of bleeding and allows for the safe removal of the overlying clot.
Although these studies are promising, aggressive removal of clot and endoscopic therapy have their own risks. Adherent clot removal is advisable in the appropriate clinical setting (high-risk patients) in the hands of expert endoscopists. It is important for the endoscopist to maintain the entire clinical picture in focus, including the appearance and location of the ulcer, before removing a clot. Large ulcers (>2 cm) and especially deep ulcers may contain exposed serosally based arteries that are expectedly large. Ulcers with these features in the posterior duodenal bulb and along the lesser curvature of the stomach may be particularly vulnerable to containing large compromised arteries—the gastroduodenal in the posterior duodenal bulb and the left gastric along the lesser curve. The caliber of these arteries may exceed the capabilities of standard endoscopic therapies. If there is question regarding the size of a potential artery underlying a dense adherent clot, endoscopic ultrasound (EUS) may be performed to screen the lesion. Preliminary data suggest that EUS-guided therapy may have an important role in delivering more effective treatment.83
Available Modalities for Endoscopic Therapy
Injection Therapy
Injection therapy is used as an adjunct with other modalities for ulcer hemostasis. Epinephrine is the most established injection agent used for peptic ulcer injection therapy. Epinephrine diluted to 1 : 10,000 or 1 : 20,000 in normal saline is found to be most effective and safest.84,85 The mechanism of action is thought to be vasoconstriction, platelet activation, and stimulation of the coagulation cascade.86 In addition, a tamponade effect resulting from the volume of fluid injected into the ulcer base is thought to be therapeutic.87
Technique
A disposable injection needle with a retractable tip is used. Injection is undertaken in 0.5- to 1.0-mL increments in all four quadrants around the nonbleeding stigmata because the path of the exposed artery is unknown. A total volume of 20 to 45 mL may be injected to achieve the desired results.88,89 No cardiac side effects have been reported as a result of larger volume of epinephrine being injected. In the setting of active bleeding, if the initial injection results in noticeable slowing and, more so, cessation of bleeding, a single injection site may suffice. Additional volume may be injected via a single site as long as any lifting effect does not impair access to the bleeding vessel for second-line therapy with coaptive coagulation or mechanical closure.
Thermal Coaptive Therapy
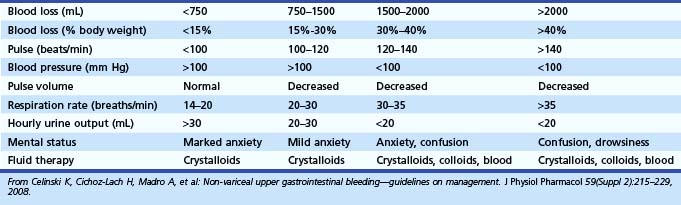
Thermal methods formerly included the neodymium:yttrium-aluminum-garnet (Nd:YAG) laser and presently include heater probe and electrocoagulation (Fig. 12.2). Laser therapy and monopolar coagulation are no longer popular because of the lack of portability to bedside, high cost, and perforation risks. Multipolar electrocoagulation and heater probe are the most widely used thermal modalities. The advantages of these devices are their excellent efficacy; safety; portability; and ability to combine irrigation, tamponade, and coagulation.
In coaptive coagulation, the probe is used to compress physically and tamponade the bleeding vessel, followed by thermal energy sealing the walls of the vessel. In an animal model, arteries with a diameter of 2.5 mm can be coagulated with a heater probe using this technique.93
Technique
Bipolar Electrocautery (Multipolar Probe)
The technique for applying bipolar electrocoagulating energy has not been standardized and varies among reported clinical trials. In canine models, a large (3.2-mm) multipolar probe produced better hemostasis than a smaller (2.3-mm) probe.94,95 Laine96 recommends forcefully applying a large (3.2-mm) bipolar circumactive probe (BICAP) on a power setting of 3 to 5 for a prolonged duration, such as 14 seconds, or seven pulses of 2 seconds each. Jensen and Hirabayashi94 used a setting of 3 to 4 with 10-second pulses on a BICAP II generator. The Gold Probe (Boston Scientific, Microvasive Endoscopy, Natick, MA), which contains a gold foil–wrapped tip, has been shown in clinical trials to be effective at low power settings with longer pulse duration.97
Heater Probe
The present technique for bleeding peptic ulcers involves using the larger heater probe, firm tamponade directly on the bleeding point or visible vessel, and coagulation with at least 120 J (four pulses of 30 J each) before moving the probe.98 An additional application of energy when using the heater probe or multipolar (bipolar) probes as the probe is removed from the treatment site can reduce unwanted tissue adhesion and the inadvertent tearing of the coagulated sealant with resultant rebleeding. In a prospective study by Bianco and colleagues,99 combined therapy with epinephrine injection with bipolar probe showed better results than epinephrine alone.
Mechanical Devices
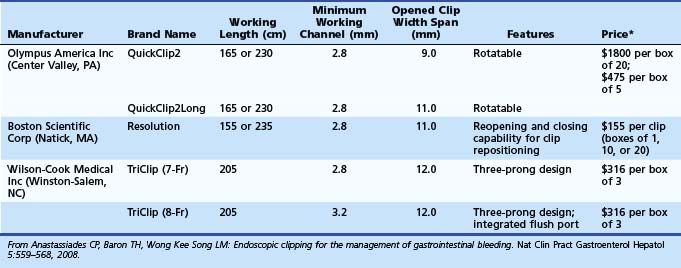
Numerous mechanical devices, including hemoclip, endoloop, and rubber band, are now available for endoscopic treatment of GI bleeding. Although endoloop and rubber band ligation have little role in treating peptic ulcer bleeding, use of hemoclips has become very popular for emergency nonvariceal hemostasis. The underlying mechanism of action is mechanical clamping of the bleeding vessel. Various clips are available (Table 12.6). Multiple randomized trials have compared use of clips alone or in combination with other therapies. Most studies have been performed on first-generation clips. Jensen and associates100 studied hemostasis capability of three presently available clips in a canine model; initial hemostasis was 100% for all types with longer retention times reported for the largest of the available clips, which can also be opened and closed on demand before deployment (see Table 12.6).
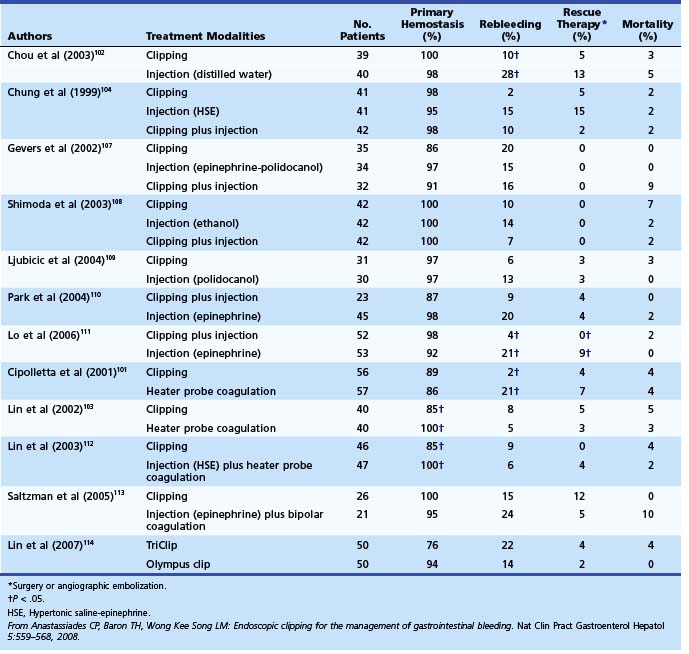
Among trials comparing hemoclip with other endoscopic therapy, Cipolletta and colleagues101 randomly assigned 112 patients with major stigmata to heater probe or hemoclip therapy (Fig. 12-3). Rates of recurrent bleeding (21% vs. 1.8%) and need for surgery (7% vs. 3.6%) were significantly lower in the hemoclip group. The investigators concluded that hemoclip therapy was safe and superior to heater probe therapy. In a randomized study by Chou and coworkers,102 hemoclip therapy was found to be superior to distilled water injection therapy in patients with major stigmata (active bleeding vessel, nonbleeding visible vessel). In their study comparing hemoclip versus heater probe, Lin and associates103 reported 85% initial hemostasis in the hemoclip group compared with 100% in the heater probe group. Rebleeding rate was 8.8% in the hemoclip group and 5% in the heater probe group. In trials comparing combination therapy, Chung and colleagues104 prospectively assigned 124 patients to hemoclip, hypertonic saline–epinephrine (HSE), and combined treatment groups. Initial hemostasis was comparable in all groups. Rates of recurrent bleed in hemoclip, HSE, and combination therapy groups were 2.4%, 14.6%, and 9.5%. Buffoli and colleagues105 did not find an additional advantage of hemoclip therapy when used in combination with epinephrine injection therapy. A favored trend toward reducing surgery was seen in the combination therapy group (0% vs. 7.4%).
Several randomized controlled trials investigating the use of endoscopic clips alone or in combination with other endoscopic modalities have reported variable success (Table 12.7).106 The most important factor seems to be difficulty in appropriate placement of the clip for effective hemostasis, especially for ulcers in difficult-to-approach sites such as high on the lesser curvature and posterior duodenal wall.103 Improvements in endoscopic clip devices have included single-use, rotatable, and reopening features. Present data suggest clips to be as effective as thermal therapy. The choice between the two modalities is at the discretion of the endoscopist depending on his or her comfort level with the device and the location of the lesion (see Table 12.7).106