Chapter 35 Colorectal Cancer Screening and Surveillance
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death in North America and Western Europe. Worldwide, there are more than 1 million new cases per year and more than 500,000 deaths. In the United States, 146,970 new cases and nearly 50,000 deaths were estimated to occur in 2009, representing about 14% of cancer deaths.1 In their lifetime, 5% to 6% of adults develop CRC.
Population-based screening can be costly and ineffective. Ideal screening employs relatively simple, inexpensive tests to risk-stratify patients followed by sensitive tests directed at individuals with highest risk. The criteria for population-based screening include factors summarized in Table 35.1. There is compelling evidence that population-based screening can reduce the incidence of and mortality from CRC.2–5 Although there is considerable variation in screening recommendations in high-risk countries, some form of screening is recommended in Europe, Canada, Australia, and the United States.
| Criteria | Colorectal Cancer |
|---|---|
| Disease is common | 5%-6% lifetime risk |
| Early detection can prevent mortality | 5-yr survival |
| Stage I: near 100% | |
| Stage II: 80% | |
| Stage III: 30%-70% | |
| Stage IV: 10% | |
| Screening methods are shown to be effective | See text |
| FOBT RCTs | |
| Sigmoidoscopy and colonoscopy case-control studies | |
| Resources are available to provide screening in U.S. | FOBT: Yes; primary care settingSigmoidoscopy: Yes; primary care or specialty clinicCT colonography: No; limited centers and fully trained radiologistsColonoscopy: Uncertain capacity (see text) |
| Resources are available to provide diagnostic tests for patients with positive screening | Colonoscopy resources are generally available if initial screening test positive |
| Screening is cost-effective | Models show cost-effectiveness |
| Screening methods are accepted by patients and providers | Yes: 50% adherence in U.S. |
CT, computed tomography; FOBT, fecal occult blood test; RCT, randomized controlled trial.
Since 1985, there has been a slow but steady decline in CRC incidence in the United States. During this time period, screening rates have gradually increased to more than 50% of the U.S. population older than 50 years. From 2000–2005, the incidence of CRC declined by 6% in men and 9.4% in women. The annual percent reduction in mortality from 2002–2004 was 4.7%.1
Epidemiology of Colorectal Cancer
Age, Gender, and Race and Ethnicity
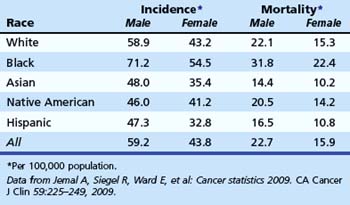
CRC represents the third most common form of cancer (excluding skin) among men and women and is the second leading cause of cancer death overall in the United States. There is a progressive increase in risk associated with age (Table 35.2). Age-adjusted incidence and mortality of CRC are higher in men. There is some evidence that natural premenopausal hormones or postmenopausal hormone replacement therapy may exert some protective effect in women.6,7
Table 35.2 Probability of Invasive Colorectal Cancer by Age and Gender
| Male | Female | |
|---|---|---|
| <40 yr | 0.08 (1/1329) | 0.07 (1/1394) |
| 40–59 yr | 0.92 (1/109) | 0.72 (1/138) |
| 60–69 yr | 1.60 (1/63) | 1.12 (1/89) |
| ≥70 yr | 4.78 (1/21) | 4.30 (1/23) |
| Lifetime | 5.65 (1/18) | 5.23 (1/19) |
| Risk of death | 2.45 (1/41) | 2.45 (1/41) |
Data from Jemal A, Siegel R, Ward E, et al: Cancer statistics 2009. CA Cancer J Clin 59:225–249, 2009.
There is strong evidence that African American men and women are more likely to die from CRC than whites (Table 35.3).1 African Americans are more likely to have advanced lesions at the time of diagnosis8 and are more likely to have proximal CRC compared with other races.9,10 The reasons for increased mortality in African Americans are unknown. Delays in diagnosis could be due to socioeconomic status or poor access to health care, although some compelling data suggest that there are biologic differences.11,12 In a study of white and African American patients undergoing screening colonoscopy, African American men and women had higher age-adjusted prevalence of polyps larger than 9 mm.8 These data support the hypothesis of biologic differences.
Lifestyle and Medications
There is strong epidemiologic evidence that lifestyle factors have an impact on CRC risk. Diets that are high in fat and low in fiber and calcium may be associated with increased risk. Obesity, metabolic syndrome, low levels of physical activity, tobacco smoking, and heavy alcohol consumption have been associated with an increased risk of CRC. There is no evidence that modification of these factors reduces CRC risk; however, changes in most of these behaviors are generally good for overall health. There is strong evidence that individuals taking nonsteroidal antiinflammatory drugs and aspirin have a lower risk of developing colon adenomas and CRC.13 However, these drugs have significant adverse effects, and are not recommended for routine CRC prevention.
Polyps and Colorectal Cancer Risk
Most CRC develops from adenoma precursors. Adenoma prevalence increases steadily with age and exceeds 35% in adults older than 75 years. There is a 5% to 6% lifetime risk of CRC, so most patients with adenomas apparently do not develop clinically detectable cancer during their lifetime. Polyp size and histology are directly associated with malignant risk. The risk of high-grade dysplasia in polyps smaller than 5 mm is 1.1%; for polyps 5 to 9 mm, the risk is 4.6%; and in polyps larger than 9 mm, the risk is 20%.14 The risk of invasive cancer is less than 1% in polyps smaller than 1 cm and greater than 10% in larger polyps.15 These data suggest that patients most likely to develop cancer are patients with advanced adenomas. Evidence from the National Polyp Study16 supports the hypothesis that detection and removal of adenomas may prevent cancer incidence. All of these data have important implications for screening. Screening efforts directed at advanced adenomas would target patients with the greatest risk and potentially lead to significant reductions in cancer incidence.
Pathogenesis
Several genetic pathways may result in the development of adenomas and CRC. In 1990, a stepwise model of chromosomal instability was proposed by Fearon and Vogelstein.17 More than 80% of sporadic CRC is associated with mutation of the adenomatous polyposis coli (APC) gene on chromosome 5. The mutation of this tumor suppressor gene most likely promotes the development of adenomas. In familial adenomatous polyposis (FAP), there is a germline mutation of this gene. Normally, this gene acts to phosphorylate beta-catenin, which leads to its destruction. Accumulation of beta-catenin leads to unregulated cell proliferation and suppression of apoptosis.18,19 The progression of adenoma to cancer seems to require additional mutations (K-ras, chromosome 18, TP53). The observation that not all tumors acquire the same mutations strongly suggests that there are several genetic pathways to malignant invasion.
A second genetic pathway (15% to 20%) of sporadic cancer is due to mutation of mismatch repair genes.20 These genes normally repair errors in DNA replication. Germline mutations are found in patients with hereditary nonpolyposis colorectal cancer syndrome (HNPCC). The key feature of this mutation in HNPCC is rapid development of polyps that progress to malignancy. In sporadic cancers, acquired mutation of these genes leads to microsatellite instability and malignant progression. Patients with large serrated adenomas in the proximal colon may have BRAF mutations and hypermethylation, which may result in microsatellite instability and progression to malignancy.21
Another germline mutation in a base-excision repair gene, MYH, has been associated with recessive inheritance of multiple colorectal adenomas. The typical phenotype is a patient with 15 or more adenomas and no germline APC mutation.22
High-Risk Groups
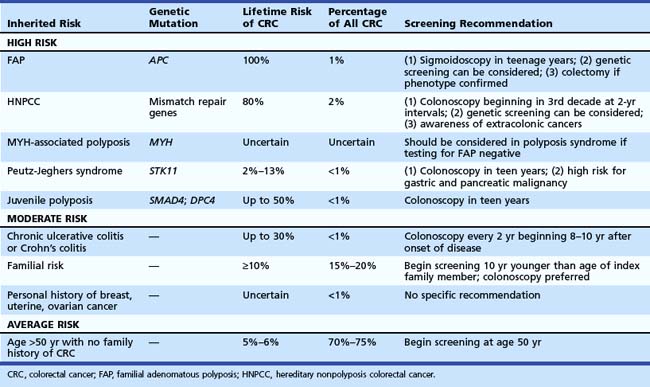
Recognition of inherited syndromes and other high-risk diseases associated with CRC is key to appropriate screening and management (Table 35.4). It is important for primary care providers to recognize these syndromes and refer patients for appropriate screening and surveillance.
Perhaps the most important questions in the medical history are the following: (1) “Do you have a first-degree relative with CRC?” (2) “If yes, did any relative have cancer before age 50 years?” Patients with an index relative younger than 50 years old should be considered at risk for an inherited syndrome and warrant intensive screening at a young age. Recommendations for specific syndromes are summarized in Table 35.4.
Inherited Polyposis Syndromes
Patients with FAP have a germline mutation of APC on chromosome 5, predisposing them to adenoma formation. Most affected patients have more than 100 adenomas. All affected individuals (100%) develop CRC, and when the phenotype is recognized, colectomy should be considered. The average age of adenoma appearance is 16 years, and the age of CRC appearance is 39 years. In a variant of this syndrome, called attenuated FAP, mutations occur at either the 5′ or 3′ end of the gene. Phenotypically, patients have fewer polyps, with delayed onset of adenoma and cancer formation. Familial colon cancer in Ashkenazi Jews may be the result of a specific germline mutation of the APC gene (I1307K). This mutation seems to predispose to sporadic mutations at distant sites resulting in a high malignant risk.19
MYH-associated polyposis leads to development of multiple polyps that may be adenomatous or hyperplastic. Mutations of the MutY human homolog (MYH) gene, which encodes a member of the base excision repair system, occur. This system normally protects cells against the mutagenic effects of aerobic metabolism. Inheritance is autosomal recessive and should be considered in patients with 10 to 100 adenomas or hyperplastic polyps who have negative genetic testing for FAP.22
Hereditary Nonpolyposis Colorectal Cancer Syndrome
HNPCC accounts for about 2% of all colon cancers. Affected individuals inherit one of several mismatch repair gene mutations. There is evidence that regular screening of kindreds can substantially reduce the risk of CRC.23 The clinical definitions have been modified over the years from the original Amsterdam criteria24 to the Bethesda guidelines,25 which provide a much less rigid clinical definition. Clinical suspicion should be high if there is a family member with CRC before age 50, multiple generations with CRC, and relatives with HNPCC-associated cancers (endometrium, small bowel, ureter, or renal pelvis). The findings of the Finnish Cancer Registry26 reinforce the need to be aware of other cancers that may develop in these kindreds, including endometrial (60%); stomach (13%); ovary (12%); bladder, urethra, and ureter (4.0%); brain (3.7%); kidney (3.3%); and biliary tract and gallbladder (2.0%).
Familial Risk
Epidemiologic data27,28 show that individuals with a first-degree relative with CRC have an increased risk of CRC. Twin studies estimated that 35% of CRC arose from inherited factors and 65% arose from environmental factors.29 A meta-analysis considered risk associated with familial risk.30 The relative risk of CRC with an affected first-degree relative was 2.4. With more than one relative, the risk was 4.2. If CRC was diagnosed before age 45 years in the index family member, the relative risk was 3.8; diagnosis at age 45 to 59 years was associated with a relative risk of 2.2. If diagnosis was made after age 59 years, the relative risk was 1.8. The risk associated with second-degree relatives is less certain because this is difficult to study. An analysis from the Utah registry found a risk of 1.5 for patients with second-degree relatives with CRC.
Based on these data, family members should be screened with colonoscopy. If the index family member had cancer after age 60 years, screening should be initiated at age 50 years with colonoscopy and performed every 10 years if colonoscopy is negative. If the family member had cancer before age 60 years, screening should be initiated with colonoscopy at age 40 and then performed every 5 years. There is also evidence that family members of patients discovered to have adenomas before age 60 years may be at increased risk for CRC,31 although this risk may be increased only if the index family member had advanced adenomas.32
Ulcerative Colitis and Crohn’s Colitis
Although patients with inflammatory bowel disease represent less than 1% of all patients who develop CRC, patients with colitis represent a high-risk group. Risk is strongly associated with extent and duration of disease. The risk is very low in the first 8 years of disease. However, in patients with pancolitis, the risk increases by up to 0.5% to 1% per year so that after 35 years of disease, the cumulative risk of CRC may be 35%.33 Patients with severe long-standing disease and primary sclerosing cholangitis may have a higher risk of developing CRC.34
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree