Chapter 40 Endoscopic Retrograde Cholangiopancreatography Tissue Sampling Techniques
![]() Video related to this chapter’s topics: Tissue Sampling of Bile Duct Stricture
Video related to this chapter’s topics: Tissue Sampling of Bile Duct Stricture
Introduction
Nevertheless, ERCP may present a unique opportunity to establish a definite diagnosis of malignancy during a drainage procedure, which may save the patient subsequent unnecessary, painful, and expensive procedures.1 Despite many years of study, imaging alone cannot make the diagnosis of malignancy.2 This chapter covers this controversial topic including historical background, pathogenesis, techniques in tissue sampling, complications, and future trends and potential.
History
Specificity in early reports was uniformly 100%. Despite early enthusiasm, clinicians noted low yields when the technique was used in a clinical setting. Reports of sensitivity of only 6% to 32% in six published studies3–8 have caused this technique to fall from practice in favor of the newer, higher yield approaches of brush cytology, fine needle aspiration (FNA) cytology, and endobiliary forceps biopsy.
Pathogenesis
Obstruction of the biliary tree by benign or malignant stricturing requiring temporary or palliative stent placement in the bile duct remains a major indication for ERCP, now that diagnostic ERCP has been largely replaced by lower risk imaging techniques of helical computed tomography (CT) and magnetic resonance cholangiopancreatography. As discussed elsewhere in this textbook, endoscopic ultrasound (EUS) has an important role in examining patients with pancreatic neoplasms in which the resectability remains uncertain after radiologic imaging. Many gastroenterologists use EUS largely to perform tissue sampling in lieu of ERCP, often because of poor yields at their center with ERCP techniques, and to permit an advance tissue diagnosis before metal stent placement. Increasingly, cross-training of endoscopists has permitted EUS and ERCP to be performed concomitantly during the same procedure to shorten hospital time, avoid repeat procedures, and reduce cost.9
These three types of malignant obstruction are pathologically distinct and represent special problems when attempting tissue sampling. The first major pathologic factor influencing biopsy or cytologic yield is tumor cellularity. Pancreatic carcinoma, in particular, often stimulates an intense desmoplastic and fibrotic reaction, making the tumor very dense and of low cellularity. Sampling often produces acellular or false-negative specimens.10,11 Maximizing yield requires repeated, deep, or large specimen sampling. Occasionally, an immune response or relative ischemia produces ulceration, bleeding, exudate, or debris that can obscure the rare malignant cell recovered in an endoscopic specimen. Cholangiocarcinoma of the primary type begins in the mucosa of the primary or secondary bile ducts. It is a relatively cellular cancer, and cells are more often shed in bile and can be more readily collected by sampling the superficial epithelium. These tumors pose difficult access problems making EUS FNA yields lower, although the procedure is technically possible in selected patients.12
Hepatocellular carcinoma often can invade and extend intraductally. Superficial sampling generally obtains diagnostic cells in this setting as well. As with pancreatic cancer, gallbladder cancer and, especially, metastatic cancer encase or compress the biliary tree, often while preserving intact benign biliary epithelium. Establishing a tissue diagnosis often requires sampling deeper than the surface epithelium.10,13–15 Very well-differentiated tumors represent a significant minority of malignant pancreaticobiliary tumors and prove very difficult to diagnose by cytologic criteria. Large specimens are often necessary to permit the pathologist to examine and compare these tumors to differentiate them from normal tissue. This fact likely explains why no biopsy technique, even open surgical wedge biopsy, has a 100% yield. These pathogenetic factors demand refined techniques and devices if adequate specimens are to be obtained to permit a positive cytologic or histologic diagnosis to be made in most cases.
Finally, there is increasing recognition of idiopathic autoimmune pancreatitis and cholangitis—a condition that can closely mimic cancer.16 This benign, IgG subtype 4–associated disorder responds to corticosteroids. It should be distinguished from malignancy if possible. A high index of suspicion for elevated IgG subtype 4 levels and negative tissue may preclude operative resection. Progress is being made to permit accurate nonoperative diagnosis of this increasingly recognized condition.17
Techniques of Tissue Sampling
Collecting adequate samples for cytologic and histologic review remains a major challenge for endoscopists during ERCP. The primary goal of planned ERCP is to provide endoscopic drainage for a patient with jaundice and obstruction; this involves obtaining ductal access, negotiating the obstruction with a guidewire, usually performing sphincterotomy, and placing a biliary endoprosthesis. Tissue sampling has always assumed a secondary position in this sequence, likely explaining the reason for the limited experience generally reported by most endoscopists. Alternatively, EUS can be scheduled with FNA as the only or at least a major goal. A cytopathologist can be present at the EUS procedure, and up to 16 needle passes have been used to establish a tissue diagnosis.18
Inadequate tissue acquisition at ERCP remains the most common reason for failing to establish an accurate pathologic diagnosis. Technical difficulty, time consideration, patient restlessness, and the need to proceed with the primary goal of biliary drainage all contribute to limit the time and thoroughness of tissue collection for many endoscopists. Because of these factors, brush cytology has been the only sampling technique adopted widely into clinical practice. Initially, standard endoscopic brushes were inserted, usually after sphincterotomy, in an attempt to sample from within a malignant-appearing stricture (Fig. 40.1A). These devices can be “groomed” by manually curving their end portions before placement. Most have a blunt smooth metal tip to minimize trauma and the risk of perforation. Nevertheless, negotiation through the stricture was often problematic. This factor and the very superficial nature of this technique of sampling produced disappointing yields, and it never became popular. Manufacturers responded to these problems by producing a variety of cytology brushes, some of which could be inserted over a guidewire placed through the malignant-appearing stricture before attempted sampling (see Fig. 40.1). Because most endoscopists concentrate on negotiating a guidewire through the stricture as the first major step in the therapeutic goal of stent placement, tissue sampling may be done at this appropriate time without changing or interrupting this sequence.
Two popular guided brushes are Combocath (Microvasive Boston Scientific, Natick, MA) and Cytomax (Wilson-Cook Medical, Winston Salem, NC) (see Fig. 40.1C). Disadvantages of these guided devices are their relative large size of 8-Fr and their stiffness. In addition, the length of the bristles must be quite short to fit within the small channel size in these double-lumen devices, a feature that may limit specimen collection. Smaller versions of these two devices have been produced but can be placed only over 0.018-inch guidewires. An alternative to using these brushes is to insert a catheter over the previously placed guidewire, withdraw the guidewire, and place a brush with a long spring-tip nose (Geenen brush, Wilson-Cook Medical) (see Fig. 40.1B). By slightly withdrawing the catheter while leaving the brush above the stricture, the tissue to be sampled can be accessed. Brushing now would not lose position above the stricture because the long nose maintains position. The principal drawback of the technique is the loss of cells when the brush is withdrawn from the catheter after the catheter is readvanced above the stricture to maintain access.19 If the entire assembly is withdrawn, reinsertion of the guidewire is required, which is an uncomfortable, and occasionally unsuccessful, challenge.
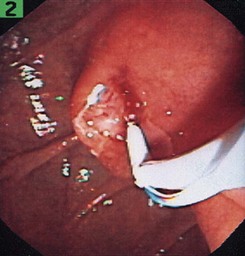
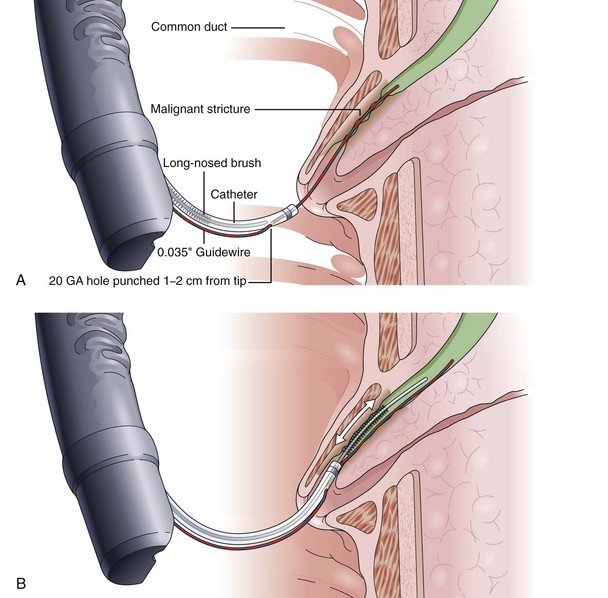
Another alternative is to create a “monorail” brushing device by piercing a catheter with a sharp 20-gauge needle 1 to 2 cm from its tip and passing the guidewire through its end hole and out this newly produced side hole. The spring-tipped brush is positioned just behind this, and the assembled device can be more easily passed into the duct (Fig. 40.2). Once above the stricture, the catheter is passed beyond the tip of the guidewire. The brush is free to advance beyond the tip of the catheter, exposing the bristles for specimen collection and leaving the guidewire in place above the stricture. The principal advantage is that the brush can be pulled into the end of the catheter, and both components can be withdrawn to minimize cellular loss (Fig. 40.3).20
Published yields of ERCP brush cytology devices vary widely for reasons that can only be speculated. Generally, series that have a higher proportion of pancreatic adenocarcinomas and, perhaps, earlier smaller tumors have a much lower yield of positive results compared with series with more cholangiocarcinomas. Published overall sensitivities using these devices range from 8% to 57%.8,21–26 As discussed subsequently, many of these series are also flawed by including patients with “suspicious for malignancy” reports as positive results. The probable pathologic explanation for these varied yields relates to the observation that the interiors of malignant strictures are composed of benign epithelium compressed by surrounding neoplastic tissue, with the exception of cholangiocarcinoma of the major bile ducts. This fact explains the low yield of simple bile aspiration for cytology because few, if any, malignant cells are in contact with the bile flow as previously discussed. When the stricture is traumatized by dilation, removing the benign epithelium, the yield of aspirating bile increases.27
The type of brush bristles, the overall brush length, and the amount of time spent brushing all affect yield. Rabinovitz and colleagues28 used three separate brushes at each ERCP and repeated the procedure with three new brushes when suspicious strictures were initially negative. Positive yield continued to increase until diagnoses were eventually made by brushing alone in 62% of their patients. Two more recent ERCP brushing studies have been performed in an attempt to increase yield. In a large number of patients, a newer long cytology brush with stiff angulated bristles was compared with the standard length brushes described previously. The true-positive yields were uniformly disappointing—only 27% and 30%—and no advantage was observed with the new brush.29 The second study compared brushing with a more traumatic technique of inserting a grasping basket through the suspicious stricture. Of 50 malignant strictures, the basket technique had a near doubling of yield to 80% compared with a brush yield of 48% (P = .018). The unexpected high yield of the brushing suggests some selection bias, and this technique requires further study.30 Additional improvements of techniques and equipment for brush cytology are needed to improve yield. Alternatively, additional techniques and devices can be used, as reviewed in this chapter.
Fine Needle Aspiration Cytology
Chiba needle aspiration cytology was pioneered in Japan in the 1950s using 22-gauge long percutaneous needles. Still the standard for most radiologically guided biopsies, Chiba needle aspiration has proved exceedingly safe and is widely applied. However, Warshaw31 reported a high rate of recovery of intraperitoneal malignant cells in patients undergoing attempted resection for pancreatic cancer who had undergone CT-guided percutaneous transabdominal Chiba needle biopsy 24 to 48 hours preoperatively. This apparent strong potential for intraperitoneal seeding has caused most physicians to seek an alternative to the Chiba technique in patients who might undergo subsequent surgery. In light of these concerns, some centers formally advocate proceeding with surgical exploration without any attempts at tissue sampling, if the clinical situation is sufficiently suspicious.32 As discussed earlier, the prospect of autoimmune pancreatitis should be considered.
Endoscopic needle biopsy of pancreatic head masses was pioneered in 1977 by Tsuchiya and coworkers33 using straight 22-gauge needles directed at bulges seen compressing the central duodenal wall. This situation represents only a small minority of such patients, but endoscopic needle biopsy remains a viable tissue sampling technique throughout the upper gastrointestinal tract for submucosal tumors.34 Intraductal FNA during ERCP required the development of a specifically designed endoscopic accessory device. Howell and colleagues26 reported on such a device after developing a ball-tipped catheter with a retractable 22-gauge Chiba-type biopsy needle (HBAN-22; Wilson-Cook Medical). The needle extends 7 mm beyond the ball tip when the catheter is placed within the duct and permits deeper sampling than afforded by brushing (Fig. 40.4). In contradistinction, intraductal FNA cytology during ERCP traverses only tissue to be resected en bloc. There can be no contamination of the peritoneal cavity including the lesser sac behind the stomach. The technique requires sphincterotomy, however, and proves to be technically challenging.
The initial high yield of 62% (positive and suspicious samples) has not been reproduced in more recent series. The true-positive sensitivity has been reported to be 27% to 30% of cases in three series.24,35,36 Nevertheless, FNA may add to the total yield when added to other techniques, as discussed later in this chapter. No complication of this technique has been reported to date.
Forceps Biopsy
The technique of forceps biopsy involves insertion of the device to the lower edge of the stricture. Using fluoroscopy, an accurate biopsy specimen can be obtained from the lower edge of the apparent tumor. Several passes of the forceps are required to produce an optimal yield. Reporting on their experience, Ponchon and coworkers13 suggested a minimum of three forceps bites.
For the technique to be practical, specialized forceps were developed. Several devices have been marketed to permit easier insertion, including two devices that are purported not to need sphincterotomy (Fig. 40.5). Easier to insert but still unguided, pediatric forceps of 5-Fr to 6-Fr work reasonably well but provide small specimens. Disposable 6-Fr pediatric forceps are now available, although they are relatively expensive (see Fig. 40.5D). Full-sized angled forceps (Maxum Carr-Locke forceps; Wilson-Cook Medical) designed to permit cannulation without sphincterotomy were also reported to be successful in a small series of patients (see Fig. 40.5B).37 This larger cup forceps has a premade angled tip but is fairly large and, in my experience, is unsuitable for cannulation except after sphincterotomy. No data on postprocedural complications have been published. A device has been marketed to enable forceps placement over a guidewire. As previously discussed, the guidewire is generally placed early in therapeutic ERCP to ensure that the major goal of biliary stent placement is successful. It is logical to use the in-place guidewire for subsequent tissue sampling.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree