Chapter 29 Nonepithelial Tumors of the Esophagus and Stomach
Epidemiology
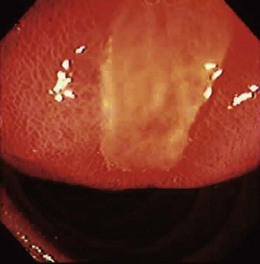
The clinical starting point for patients with lesions of nonepithelial origin is the discovery of a mass impinging on the GI tract mucosa from beneath—the so-called submucosal tumor (Fig. 29.1). In its classic form, a discrete tumorous appearance is present with overlying mucosa that, although most commonly bland, may be erythematous, pale, dimpled, or ulcerated. Lesions are often initially identified at esophagogastroduodenoscopy (EGD), but the patient may also be referred to an endoscopist for evaluation of an abnormal radiograph (e.g., barium-contrast examination).
Applied literally, the term submucosal would imply the presence of an intramural mass originating in the submucosal layer of the GI wall. However, the term has come to be used for a range of lesions that create a similar appearance, including intramural and extramural structures. Such submucosal tumors may include both neoplastic and nonneoplastic masses, and even mucosal neoplasms have been reported to exhibit a submucosal appearance.1 Examples of nonepithelial lesions in all four categories are listed in Table 29.1. This discussion centers on neoplasms that primarily originate from nonepithelial GI tract cell lines, but all types of pathology must be considered when developing a management plan.
Table 29.1 Types of Masses Causing Esophageal and Gastric Submucosal Tumors
| Neoplastic Masses | Nonneoplastic Masses | |
|---|---|---|
| Intramural masses | Stromal cell tumor | Varices |
| Lipoma | Duplication cyst | |
| Granular cell tumor | Inflammatory granuloma | |
| Lymphoma | Foreign body (e.g., surgical suture or clip) | |
| Fibrovascular polyp | Pancreatic rest | |
| Hemangioma/hemangiosarcoma | ||
| Lymphangioma/lymphangiosarcoma | ||
| Metastatic neoplasm | ||
| Extramural masses | Primary neoplasm of adjacent organs (benign and malignant) | Benign lymph node |
| Metastatic lymph node | Inflammatory mass of adjacent organs (e.g., pancreas, spleen) | |
| Organomegaly (e.g., spleen, liver) |
Depending on the clinical circumstances and type of tumor, the lesion may cause symptoms such as bleeding, obstruction, or pain. However, such lesions commonly are serendipitously found during evaluation for a different, unrelated problem. Because most such lesions are asymptomatic, epidemiologic data are skewed by the nature of their discovery incidental to a different, usually unrelated condition. In one study of 15,104 EGD reports, submucosal tumors were identified in 0.36%.2 Because most in this series were life-threatening tumors, the study database likely underreported less serious lesions. Many such lesions turn out to be normal extramural organs. Allgayer3 found that among 30 patients referred for submucosal tumors, normal extramural structures were present in 14 (47%). Motoo and coworkers4 also reported normal organs in 16 of 19 submucosal tumors, as did Caletti and colleagues5 in 10 of 25 tumors; organs identified include the spleen, liver, splenic vessels, and pancreas.
Because submucosal tumors are often left in situ, the pathologic distribution among tumors is unknown. It is reported that 1% to 3% of resected gastric tumors are stromal cell tumors6; it can be inferred that the actual incidence, when including tumors that were not resected, is considerably higher. In a small prospective study,7 among 45 submucosal tumors, most were found to have a benign appearance that required no follow-up. From these available data, it may be cautiously concluded that submucosal tumors are found in less than 1% of routine upper endoscopy examinations, half of such lesions are found to be normal extramural structures, most remaining lesions are benign, and stromal cell tumors constitute most such neoplasms.
Clinical Features
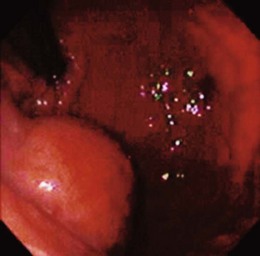
Lumps and bumps of all sorts are regularly encountered in endoscopic examinations; the decision regarding which to evaluate further depends on the endoscopic appearance, the clinical circumstances, and the inclination of the endoscopist. Because few standardized guidelines exist to direct such decisions, great variation in practice exists. Symptoms attributed to the mass nearly always drive further investigation, but our own endoscopic ultrasound (EUS) study of a subset of such lesions found that nearly 90% were asymptomatic.8 GI bleeding may be seen in many submucosal lesions, most commonly in the form of slow blood loss causing iron deficiency anemia. The surface of the tumor may be ulcerated in such cases (Fig. 29.2). Malignant tumors may be more prone to ulceration and bleeding9; this might be taken as a sign of a potentially malignant form that compels definitive treatment. However, benign lesions may also cause severe bleeding,10 and occasionally rapid hemorrhage may occur.11 Less often, GI tract obstruction may be caused by such masses,12 especially if the lesion is located in a narrow area such as the esophagogastric junction or pylorus; intussusception caused by such masses has been reported.13 Pain may be a presenting complaint, especially if the submucosal tumor is neoplastic or malignant.14
Because most lesions are incidentally found during endoscopic examination for another problem, the clinical features of submucosal masses are primarily those that, in the endoscopist’s opinion, compel further evaluation. Large size has been proposed as an ominous finding,15 and lesions with an ulcerated or irregular (lumpy) surface often undergo additional testing or treatment. Patients with submucosal tumors who have a prior history of malignancy should receive further evaluation to exclude metastatic disease. Finally, patients with submucosal lesions that change appearance on serial examination are usually directed by an alert clinician to further testing.
Pathology
Extramural masses compose half of suspected submucosal tumors and include normal organs, nonneoplastic masses, and extramural neoplasms. Normal liver, spleen, pancreas, gallbladder, colon, and kidney all have been reported to appear as suspected submucosal tumors.3–5 Vascular structures often produce the appearance of a discrete tumor, including normal vessels of the spleen16 and abnormal vessels such as varices and aneurysms.17 Neoplasms and nonneoplastic masses involving these same organs can also produce this appearance, as can such masses involving the peritoneum, mediastinum, and the lymph nodes adjacent to the upper GI tract. The various malignancies, cysts, and inflammatory masses of these structures need no further elaboration here because a large variety of such findings have been noted in the case report literature.
Masses that arise within the wall of the esophagus and stomach require further discussion, particularly because many are peculiar to the GI tract. Most neoplasms in this category are mesenchymal tumors, meaning that they arise from cells of mesodermal origin. Most such neoplasms are clinically benign, although, as shown subsequently, tumor histology may not provide reliable clues to malignant behavior. A large variety of such neoplasms have been described (Table 29.2), but most are exceedingly rare. The tumors most likely to be encountered in the esophagus and stomach in a routine clinical setting are discussed here.
Table 29.2 Classification of Gastrointestinal Mesenchymal Tumors
| Tumor Type | Examples |
|---|---|
| Stromal tumors | Smooth muscle tumors (leiomyoma, leiomyosarcoma), glomus tumors, leiomyomatosis, pleomorphic sarcoma |
| Neural tumors | Neuroma/neurofibroma, paraganglioma, ganglioneuromatosis |
| Endothelial and vascular tumors | Hemangioma, hemangiosarcoma, Kaposi’s sarcoma, lymphangioma |
| Lipocytic tumors | Lipoma, liposarcoma, lipohyperplasia (ileocecal valve), lipomatosis (colon) |
| Granular cell tumor | Granular cell tumor |
| Inflammatory fibroid polyp | Inflammatory fibroid polyp |
| Fibrohistiocytic tumors | Fibrovascular polyp, fibrous histiocytoma, desmoid tumors (mesentery), fibroepithelial polyp |
| Striated muscle tumors | Rhabdomyosarcoma |
Data from Lewin K, Riddel RH, Weinstein WM: Mesenchymal tumors. In: Gastrointestinal pathology and its clinical implications, New York, 1992, Igaku-Shoin, pp 284–341.
Gastrointestinal Stromal Tumor
Most mesenchymal GI tumors are pale, firm, spherical, or ovoid structures embedded in the wall of the affected organ. The microscopic appearance of musclelike eosinophilic, spindle-shaped cells in uniform sheets and the proximity of the tumors to the muscular wall layers led early observers to believe that these tumors were of myogenic origin18—hence the name leiomyoma and its variations (e.g., leiomyosarcoma, leiomyoblastoma). However, it later became clear that these neoplasms not only are not of obvious myogenic origin but also often lack any specific markers of differentiation whatsoever.19 Immunohistochemical analyses showed variable expression of smooth muscle features such as desmin and actin and neural proteins such as S-100.20 For the sake of clarity, these lesions came to be referred to as gastrointestinal stromal tumors (GISTs), an acknowledgment that they originate in mesenchymal stroma.
A significant breakthrough occurred with the discovery that most GI stromal tumors stain positive for a specific membrane protein, designated CD117,21 and subsequently identified as KIT, a tyrosine kinase receptor.22 Tyrosine kinases are a class of transmembrane receptors that mediate various cellular growth functions. The extracellular portion of KIT binds stem cell factor, causing a conformational change. The receptor subsequently forms a dimer with another activated KIT receptor, and the intracellular region of the resulting dimer triggers various cell signaling cascades.23 Among these are proliferation pathways such as mitogen-activated protein kinase (MAPK) and signal transducers and activators of transcription (STAT) proteins as well as antiapoptotic mediators such as AKT (protein kinase B).24 KIT and some other tyrosine kinases are important regulators of cell growth and proliferation.
Abnormal cell growth is a fundamental element of cancer physiology, and hyperfunction of the KIT receptor can lead to neoplasia. In this case, numerous gain-of-function mutations in the KIT gene have been described.25 These acquired (or, rarely, inherited) mutations produce KIT receptors that are capable of initiating the aforementioned intracellular growth cascades without activation by stem cell factor. The resulting dysregulated growth seems to be an important initial step to tumorigenesis. This concept is supported by the observation that nearly all GISTs express KIT. It has further been observed that the interstitial cells of Cajal (ICC) share some phenotypic and ultrastructural similarities with GIST and normally express the KIT receptor; this observation has led to the current hypothesis that GISTs arise from ICC26 or from ICC precursor cells. Finally, it has been noted that this gain-of-function mutation is not found in true leiomyomas.27 Some pathologists have suggested that CD117 positivity is required to confirm a diagnosis of GIST.28 It is now known, however, that a few otherwise obvious GISTs do not express KIT. Many of these tumors have been identified as expressing platelet-derived growth factor receptor α (PDGFRα), another distinct tyrosine kinase receptor that also mediates many of the same growth and antiapoptotic proliferation pathways.29 Several gain-of-function mutations have been reported in the PDGFRα gene, and the resulting tumors are otherwise indistinguishable from GISTs that are KIT-positive.
Nearly all upper GI tumors of this type occur in the stomach, but duodenal lesions have been described.30 Most esophageal stromal tumors lack the CD117 protein and may be true leiomyomas.28 The endoscopic appearance is a dome-shaped, firm submucosal mass; central umbilication or frank ulceration is common, and there may be a lobulated or irregular appearance. The tumors are most often solitary except in the case of specific disease entities such as Carney’s triad (GIST, pulmonary chondroma, and extraadrenal paraganglioma). Germline KIT mutation kindreds have been described,31 however, in which case multiple GISTs are seen. Giant sizes of greater than 10 cm have been noted, but most tumors are less than 3 cm.
Pathologically, the tumor usually consists of uniform pale tissue, although hemorrhagic and necrotic areas may be seen. Microscopically, the cells are spindle-shaped with uniform nuclei and general cytologic uniformity. Some cell groups may show epithelioid configurations (closely packed polygonal cells), and there may be nuclear pleomorphism. It has been observed more recently that the histologic pattern is sometimes related to the nature of the underlying genetic abnormality; KIT mutations at exon 13 or 17 more often show spindle-cell morphology,32 whereas PDGFRα GISTs often exhibit epithelioid histology.33 Ultrastructural cellular abnormalities have also been linked to specific gene mutations.34 However, malignant behavior has not been associated with specific mutation patterns.
It has long been known that malignant behavior in GISTs is difficult to predict given the relatively bland cytology and slow growth of these neoplasms. It has been reported that even small, benign-appearing stromal tumors have been known to metastasize.35 This discovery led to considerable confusion about the appropriate criteria to categorize these tumors as benign or malignant. Older pathologic scoring systems relied on numerous histologic features19 and were plagued with problems. More recent attention has focused on the size of the tumor and the number of mitoses observed (mitotic index), at least in part because these are easily quantifiable findings. In one study of 100 cases, tumors with more than 5 mitoses per 10 high-power fields (HPFs) were significantly more likely to metastasize, although 40% of malignant lesions in that study had fewer mitoses.36 In another study, multivariate analysis of various clinical and pathologic features in 122 specimens showed that more than 10 mitoses/50 HPFs correlated with poor outcome, whereas site, epithelioid histology, and tumor size were not independently predictive.37
Attempts to correlate tumor marker status such as CD117 positivity with malignant behavior have produced generally confusing or negative results,38 as have studies of specific KIT mutations27,39 and other tumor markers.40,41 Older GIST scoring systems have been abandoned following a National Institutes of Health (NIH) consensus conference, which defined malignancy risk based on size and mitotic index alone.42,43 The NIH criteria divide tumors into four categories of malignant risk (Table 29.3), an acknowledgment that even the most innocent lesion poses a slight but definite risk of malignant behavior.
Table 29.3 National Institutes of Health Criteria for Malignant Risk in Gastrointestinal Stromal Tumors
| Risk Level | Size (cm) | Mitoses/50 HPF |
|---|---|---|
| Very low risk | <2 | <5 |
| Low risk | 2–5 | <5 |
| Intermediate risk | <5 | 6–10 |
| 5–10 | <5 | |
| High risk | >5 | >5 |
| >10 | Any | |
| Any | >10 |
HPF, high-power field.
Data from Berman JJ, O’Leary TH: Gastrointestinal stromal tumor workshop. Hum Pathol 32:578–582, 2001; Toquet C, Le Neel JC, Guillou L, et al: Elevated (> or = 10%) MIB-1 proliferative index correlates with poor outcome in gastric stromal tumor patients: A study of 35 cases. Dig Dis Sci 47:2247–2253, 2002; Hedenbro JL, Ekelund M, Wetterberg P: Endoscopic diagnosis of submucosal gastric lesions: The results after routine endoscopy. Surg Endosc 5:20–30, 1991.
If all such neoplasms entail malignant risk, prudence might dictate that they should always be resected. However, more recent data suggest that GISTs are more common than previously thought. Up to 10% of resection and autopsy specimens contain such tumors,44 and microscopic GISTs (also called seedling GISTs, minimal GISTs, or GIST tumorlets) can be seen in 35% of some patient groups.45,46 These incidental and microscopic GISTs display the same KIT and PDGFRα mutations as their larger counterparts. The finding that synchronous GISTs from the same patient regularly show different gene mutations (and are independent sporadic GISTs) confuses the picture further.47 GISTs that were earlier classified as metastatic or recurrent may have been distinct neoplastic events. If it turns out that most small and asymptomatic GISTs stay that way, a conservative approach may be the most prudent.
A second breakthrough in GISTs has been the development of tyrosine kinase inhibitors such as imatinib mesylate that are effective in reducing the KIT enzyme activity and that are useful for tumor treatment. Imatinib targets the specific abnormal enzyme activity in the neoplasm and does not rely on generalized cytotoxicity for its effect. In an open label study of 147 patients with unresectable malignant GISTs, an overall response rate of 38% was seen.48 Among responders, results are often dramatic (Fig. 29.3). The recognition of the malignant potential of GIST, combined with the availability of effective treatment even for unresectable disease, has compelled new thinking in the accurate diagnosis of this neoplasm.