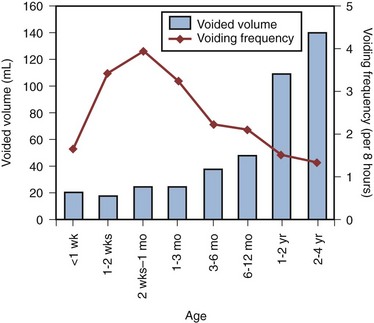
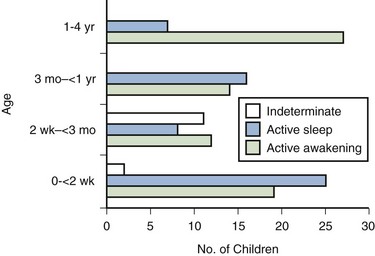
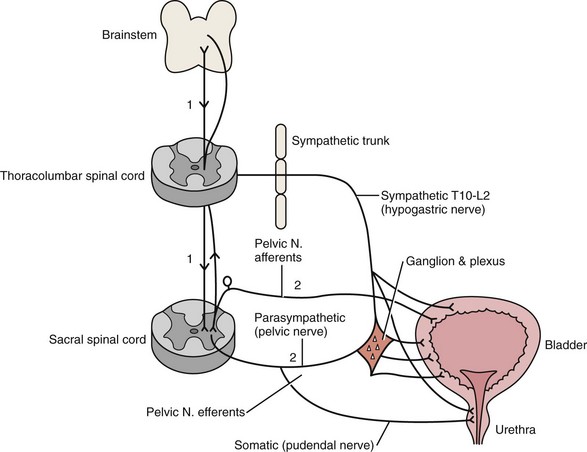
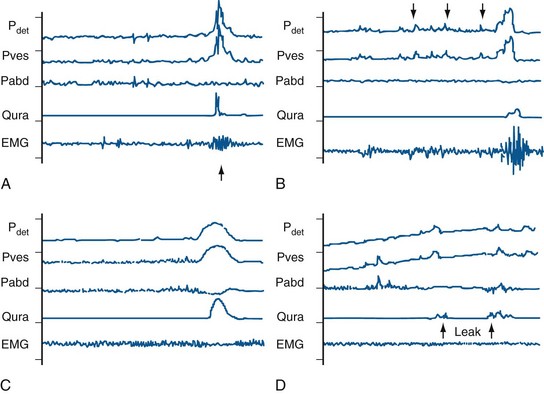
C.K. Yeung, MD, Jennifer D.Y. Sihoe, MD Our increasing understanding and recognition of non-neuropathic lower urinary tract dysfunction as a cause of various urologic disorders in childhood has had a profound influence on our management strategies over the past decade. What was known as primary or idiopathic, such as primary vesicoureteral reflux (VUR) or primary nocturnal enuresis, is often associated with underlying bladder dysfunctions evident on urodynamics and resolves with successful correction of the bladder physiology (Koff and Murtagh, 1983; Homsy et al, 1985; Watanabe et al, 1994; Yeung et al, 1998, 1999). Conversely, failure of recognition and treatment of the associated problem can result in persistence or even further deterioration. The understanding of the spectrum of dysfunction of the bladder and even of normal bladder physiology in infants and young children is complex. Not only are the dynamics and functional disturbances of the lower urinary tract different from those in adults, but the evolution in normal bladder-sphincteric function during growth and maturation in children poses continuous changes (Yeung, 1995; Yeung et al, 1995; Sillen et al, 1996; Holmdahl, 1997). More confusingly, one type of bladder dysfunction may often progress with time and evolve imperceptibly into another, without a sharp distinction between the different stages. This is further confounded by a lack of age- and sex-specific normal reference values for various urodynamic parameters, especially for the very young age groups (Yeung et al, 1995). Recent advances and development in the use of urodynamic techniques specially designed for infants and young children have allowed more accurate assessment of bladder function and provided much better understanding of bladder pathophysiology. This has allowed for better recognition of functional disorders, as well as to provide a scientific basis for their therapy (Hjalmas, 1988; Perez et al, 1992; Bauer, 1997; Nevéus et al, 2007). In view of the important association of bladder dysfunctions with various common urologic disorders, urologists should be acquainted with the spectrum of dysfunction and the techniques to facilitate proper diagnosis and treatment. The bladder is a unique organ of the human body in that it not only carries a dual function of both storage and emptying of urine but also has a complex innervation of voluntary and involuntary control of function. Understanding of the functional anatomy of the lower urinary tract stems from extensive postmortem studies carried out over decades (Gosling et al, 1981; Gosling, 1985; DeLancey, 1988; de Groat, 1993; Zvara et al, 1994). The bladder is an abdominal organ, and when full it can be readily palpable in infants and young children due to a shallow pelvis (Wiegel, 1990). The bladder wall consists of three layers: mucosa, detrusor, and adventitia. The detrusor consists of a meshwork of smooth muscle fibers arranged into a single functioning unit with an ability to elicit nearly maximum active tension over a wide range of length. This allows the bladder to fill with urine from the upper tract at low pressures (compliance) (Mattiasson, 1994). The ability of the bladder to store urine (reservoir function) is determined by the concomitant activity of the detrusor muscle and the bladder outlet (consisting of the bladder neck, proximal urethra, and striated muscle of the pelvic floor) (de Groat, 1993). Little, too, is known about the natural course of development or maturation of the structure and function of the sphincter mechanism. Literature suggests that immature detrusor-sphincter coordination, manifested as detrusor hypercontractility and interrupted voiding, commonly occurs in the first 1 to 2 years of life, causing some degree of functional bladder outflow obstruction (Sillen et al, 1992; Yeung et al, 1998). In a postmortem study of the ontogeny of the external urinary sphincter in human fetuses, infants, and young children, Kokoua and Homsy found significant age-related differences in the histologic structure of the sphincter as compared with that in adults. Striated muscle fibers of the sphincter first appeared at around 20 weeks of gestation and then became arranged in a concentric pattern as a closed ring, fused posteriorly to form a tail-like structure that was directed to the perineal body. Posterior splitting of the striated sphincter, starting first caudally and progressively in a cephalad manner, then occurred during the first year of life, coinciding in parallel with gradual resorption of the “tail,” to eventually giving way to a mature omega-shaped structure (Kokoua et al, 1992). As a complete closed ring of striated sphincteric muscle was present up to 1 year of age in more than 40% of cases, it may well be conjectured that this could be related to the high intravesical pressures and interrupted voiding that are commonly observed during urodynamic studies in infants (Sillen et al, 1992; Yeung et al, 1995, 1998). Activation, coordination, and integration of various parts of the bladder-sphincteric complex involve both the central somatic and autonomic nervous systems through three sets of peripheral nerves: sacral parasympathetic (pelvic nerve), thoracolumbar sympathetic (hypogastric nerves and sympathetic chain), and sacral somatic nerves (primarily the pudendal nerve) (Fig. 127–1) (deGroat, 1993; Mattiasson, 1994). Figure 127–1 Diagram to illustrate the innervation of the bladder-sphincter complex. (From Yeung CK, Barker GM, Läckgren G. Pathophysiology of bladder dysfunction. In: Gearhart JP, Rink RC, Mouriquand PDE, editors. Pediatric urology. 2nd ed. Philadelphia: Elsevier Saunders; 2010. p. 353–65.) Parasympathetic nerve fibers run in the pelvic nerve (S2-S4) to supply the pelvic and vesical plexuses before entering the bladder. Parasympathetic ganglia are found within these plexuses and in the bladder wall. Sympathetic nerves arise from segments T10-L2 of the spinal cord and go to the inferior mesenteric ganglion through the sympathetic trunk. From the inferior mesenteric ganglion the nerve fibers pass to the pelvic plexus and bladder through the hypogastric nerves. There is also sympathetic innervation originating from T10-L2 supplying the detrusor and urethral sphincter (Bradley et al, 1974). The somatic nervous system (pudendal nerve) supplies the periurethral pelvic floor musculature (Mattiasson, 1994). The sensory and motor nerves carried by all three nerves innervate both the bladder and urethral sphincter. They originate from parasympathetic ganglia located in the second, third, and fourth segments of the sacral spinal cord (Bradley et al, 1974). Within the spinal cord, information from bladder afferents is integrated with that from other viscera and somatic sources and projected to the brainstem centers that coordinate the micturition cycle (Harrison and Abrams, 1994). Urodynamic studies on normal infant bladders have shown that bladder function in young children is different from that in adults. During the first 2 to 3 years of life there is progressive development from an initially indiscriminate infantile voiding pattern to a more socially conscious and voluntary or adult type of micturition. This is achieved through an active learning process whereby the child acquires the ability to voluntarily inhibit or initiate voiding at socially convenient times. This natural evolution of bladder control entails an intact nervous system and depends on at least three main events occurring in parallel: (1) a progressive increase in bladder functional storage capacity; (2) maturation of voluntary control over the urethral striated muscle sphincter; and, perhaps most importantly, (3) development of direct volitional control over the bladder-sphincteric unit so that the child can voluntarily initiate or inhibit the micturition reflex. This process can also be influenced by an awareness of the accepted social norms in families during toilet training (Yeung et al, 2010). During the third trimester of pregnancy, the fetus is voiding at the rate of approximately 30 times every 24 hours (Goellner et al, 1981). However, immediately after birth, this drops dramatically for the first few days of life, only to increase again after the first week to reach a peak by weeks 2 to 4 to an average of once per hour. Subsequently this rate declines again to about 10 to 15 times per day between 6 and 12 months and to about 8 to 10 times per day by 2 to 3 years (Goellner et al, 1981; Yeung, 1995; Holmdahl et al, 1996). This reduction in voiding frequency observed during the first few years of life appears to be related mainly to an increase in bladder capacity in parallel to body growth, which is proportionately greater than simultaneous increase in urine volume production (Yeates, 1973; Koff, 1997). By the age of 12, the voiding pattern is similar to that in an adult and usually comprises 4 to 6 voids per day (Fig. 127–2). For older children, one of the most widely accepted formulas is the Koff formula: Or similarly, the Hjalmas formula follows: In parallel to the increase in bladder capacity, the mean voided volume of each micturition increases with age. Of note, urodynamic studies have shown that a significant proportion of infants with incomplete maturation of detrusor-sphincter coordination before the age of 1 are still able to achieve satisfactory bladder emptying (>80% efficacy) (Yeung, 1995; Yeung et al, 1995, 1998; Holmdahl et al, 1996; Bachelard et al, 1999; Sillen et al, 2000). Studies on detrusor pressures at voiding in normal infants are limited due to (1) the technical difficulties involved in performing urodynamic studies in young infants and (2) an ethical consideration in justifying doing so. From the data in a natural filling cystometric study of infants with normal lower urinary tracts (as indicated by a normal micturating cystourethrogram) and who had undergone either dismembered pyeloplasty for pelviureteric junction obstruction or nephrectomy for dysplastic kidney, the authors have documented significantly higher maximum detrusor pressures with micturition (Pdetmax) than in normal adults. It was also noted that male infants voided with significantly higher pressures than females (mean Pdetmax: 118 versus 75 cm H20 respectively, P < .03) (Yeung et al, 1995a, 1995b, 1998). Similar findings were reported in healthy, asymptomatic infant siblings of children with VUR (Bachelard et al, 1999). Studies have also shown that these high detrusor pressures noted during micturition were mainly observed only during the first year of life and decreased progressively with age. Furthermore, an interrupted or “staccato” type of urinary stream was noted in more than half of the patients (Yeung et al, 1995a, 1995b, 1998). This was demonstrated by fluctuations of the detrusor pressure when it reached maximum during voiding and resumption of urinary stream in conjunction with a sharp fall in the detrusor pressure. The high detrusor pressures during voiding are thought to represent variations among individual infants in the maturation process of detrusor and sphincter coordination during the first 1 to 2 years of life (Yeung et al, 1995, 1998; Holmdahl et al, 1996; Bachelard et al, 1999; Sillen et al, 2000). The authors have further confirmed this finding using video cystometry under fluoroscopy combined with natural fill urodynamics and perineal electromyography (EMG) in infants with a history of urinary tract infections (UTIs). Periods of increase in perineal or sphincteric EMG activities were noted during voiding and associated with a sudden cessation of urinary flow with a simultaneous isometric rise or high peak of detrusor pressure. In contrast, resumption of urinary flow was associated with relaxation of the external urinary sphincter and a paradoxical drop in detrusor pressure. Also, the detrusor pressure associated with the initiation of urinary flow was usually significantly lower than the maximal detrusor pressure during micturition (Pdetmax) and that the Pdetmax was significantly higher than those recorded in normal adults (Fig. 127–3). (From Yeung CK, Barker GM, Läckgren G. Pathophysiology of bladder dysfunction. In: Gearhart JP, Rink RC, Mouriquand PDE, editors. Pediatric urology. 2nd ed. Philadelphia: Elsevier Saunders; 2010. p. 353–65.) Traditionally, it has been assumed that micturition in newborns and young infants occurs automatically with a full bladder by a simple spinal cord reflex, with little or no mediation by the higher neural centers, and that with progressive maturation, voluntary inhibition of the bladder emptying reflex is achieved by adulthood. A delay in the normal maturation of bladder control was attributed to certain conditions such as primary nocturnal enuresis and hence the traditional belief that all enuretics would get better with age (Nash, 1949). However, more recent studies have indicated that this is an oversimplification of what actually occurs. Even in full-term fetuses and newborns, it has been shown that micturition is modulated by higher centers. Ohel and colleagues (1995) showed that intrauterine micturition almost exclusively occurs while the fetus is awake rather than randomly distributed over various behavioral (sleep/arousal) states. Furthermore, it has been observed that micturition in a full-term fetus can be elicited by vibroacoustic stimulation, all of which indicates that the micturition reflex is probably under higher neural control even at near gestational term (Zimmer et al, 1993). Further extensive modulation occurs during the postnatal period. Studies on normal neonates using ambulatory bladder monitoring techniques in conjunction with polysomnographic recordings have shown that even in newborns, micturition does not occur during sleep (Yeung et al, 1995). During sleep the bladder is normally quiescent and stable with lack of facilitation of detrusor contractions, whereas during wakefulness marked detrusor overactivity is observed. Clear electroencephalographic (EEG) evidence of cortical arousal or actual awakening occurs in response to bladder distension, and sleeping infants are noted to wake up before bladder activity returns and voiding occurs. However, this arousal period may often be transient with the infant crying or moving for a brief period, micturating, and then going back to sleep without being noticed to have awakened. This wakening response to bladder distension probably involves more complicated neural pathways and higher centers than has been appreciated up until now (Fig. 127–4). These results also correlate with recent animal studies showing a sophisticated integration of preexisting central and peripheral neural pathway in micturition control at birth with remodulation occurring in the early postnatal period (Maggi et al, 1986; Thor et al, 1989). Extensive studies using experimental animals by de Groat and colleagues (1993, 1998) have indicated that early postnatal maturation of bladder function probably occurs at different levels: (1) changes in the properties of detrusor muscle; (2) developmental modifications in the peripheral innervation of the bladder; and (3) alterations in central synaptic circuitry and neuroplasticity in the parasympathetic reflex pathways to the bladder (Sugaya and de Groat, 1994; Araki and de Groat, 1997; Sugaya et al, 1997). Recordings of spontaneous activity in bladder smooth muscle in neonatal rats showed much larger amplitude and more synchronous rhythmic contractions compared with those observed in adult rats (Sugaya and de Groat, 1994). This suggests that there is a progressive reduction in intercellular communication between detrusor smooth muscle cells, resulting in less spontaneous activities and hence more efficient urine storage, during early postnatal development. In addition, peripheral and central neural mechanisms also change extensively during this period. In cats (and some other species) micturition during the newborn period is dependent on an exteroreceptive somatovesical reflex triggered when the mother licks the perineum of the kittens (de Groat et al, 1993, 1998; Araki and de Groat, 1997). This somatovesical reflex, processed in the sacral spinal cord, disappears in older animals but may reappear after spinal cord injury. Further neuroanatomic studies have indicated that spinal bladder reflexes are mediated via interneurons located immediately adjacent to, and synapsing with, the sacral preganglionic neurons (Sugaya et al, 1997). This interneuron–preganglionic neuron synaptic transmission is efficient immediately after birth but is abruptly downregulated during the third postnatal week when the mature supraspinal micturition reflexes start to appear (Araki and de Groat, 1997). Transection of the spinal cord prevents this downregulation, indicating that the higher neural centers play an important role in this synaptic remodeling, which contributes to postnatal development of micturition reflexes. Neurologic control of normal micturition occurs at different levels of the central nervous system (CNS), from the spinal cord with the “sacral micturition center,” to the brainstem with the “pontine micturition center,” the cerebellum, basal ganglia, limbic system, thalamus and hypothalamus, and cerebral cortex (Blaivas, 1982; McLorie and Husman, 1987; de Groat, 1993; Fernandes et al, 1994). It should be noted that the bladder is unique among other visceral organs in that its function is under the control of both the somatic and the autonomic nervous system. Besides acetylcholine and norepinephrine, various other neurotransmitters including prostaglandin substance P, opioid peptides, vasoactive intestinal peptide, and neuropeptide Y are involved during bladder stimulation (Fernandes et al, 1994). Simple manipulation of adrenergic and cholinergic receptors may only partially abolish the effect of neural stimulation, which explains why pharmacologic blockage of the classic neurotransmitters (acetylcholine and norepinephrine) alone may fail to elicit the expected full clinical response. Studies have shown that in making the transition from an infantile to an adult pattern of micturition control, all children may transiently display some degree of abnormal bladder-sphincteric function (Koff, 1997). For instance, it has been clearly shown that a significant proportion of normal infants exhibit prominent detrusor-sphincter discoordination and interrupted voiding during the first 1 to 2 years of life. This is manifested by a discoordinated and interrupted urine flow, which may even be brought to a complete stop for 1 to 2 minutes before restarting, producing a pattern of repeated small voidings in quick succession (Yeung et al, 1995a, 1995b, 1998). Urodynamic findings show association with high voiding pressures and interruption of flow but no impairment of overall bladder emptying. However, this type of dysfunction resolved with a period of successful toilet training and was only transitory or intermittent and did not persist. One must therefore be cautious in the assessment of young children with apparent voiding dysfunctions and resist the temptation to overinterpret intermittent or transient symptoms as pathologic and hence overinvestigate. However, if voiding dysfunctions are persistent well beyond the period of toilet training, especially if associated with urinary complications like recurrent urosepsis, then the possibility of underlying anatomic and neurologic causes must be considered and duly evaluated. Key Points: Normal Bladder Function and Development in Infants and Children Non-neuropathic bladder-sphincter dysfunction in children is probably more common than what meets the eye, but many times it only presents to us when UTI, VUR, or urinary incontinence is manifested. It has been reported that 15% of 6-year-old children suffer this condition (Hoebeke, 2002). Various conditions have been described under the category of non-neuropathic bladder-sphincter dysfunction, but it must be emphasized that these conditions should not be viewed rigidly as separate and distinct entities but rather as transitional phases of a complex sequence of events. For instance, a girl with dysfunctional voiding may start with having detrusor instability associated with sphincter and pelvic floor overactivity, then gradually evolve to develop fractionated voiding with increasing postmicturition residues, and finally develop bladder decompensation and the “lazy bladder” syndrome (van Gool et al, 1992). It must also be emphasized that use of the term “non-neuropathic” is based purely on the fact that no obvious and identifiable neurologic lesions can be identified. However, conditions like the urofacial syndrome complex (Ochoa syndrome) and the Hinman syndrome behave almost identically to the typical neuropathic bladder-sphincter dysfunctions. It is indeed conceivable that they do have an organic underlying neurologic cause, although the exact neuroanatomic lesion has not yet been identified. Hence the distinction between neuropathic and non-neuropathic bladder dysfunctions may not be as clear as traditionally thought. In adults, lower urinary tract function has been well understood and standardization of terminology has been established by a committee working under the International Continence Society (ICS) since the early 1970s (Bates et al, 1976a, 1976b, 1979). In contrast, neural control over the bladder-sphincter unit in children is age dependent and is much more variable and complex. Definitions of normal versus abnormal lower urinary tract function have therefore been far less standardized. Various classifications for bladder dysfunctions in children have been described over the past few decades (Bellinger, 1996; Lapides, 1970; Wein, 1998). To avoid confusion, the classification proposed by the International Children’s Continence Society in 2007, with a standardized set of terminology and definitions for different lower urinary tract dysfunctions, was adopted (Nevéus et al, 2007). Dysfunction of the lower urinary tract in children can be secondary to derangement of nervous control, disorders of detrusor and sphincteric muscle function, structural abnormalities, and other unclassified conditions (Nevéus et al, 2007). This is based on the functional state of the bladder-sphincteric complex with respect to detrusor activity, bladder sensation, bladder compliance and capacity, and urethral function both during the filling and the voiding phase of cystometry (Fig. 127–5) (Nevéus et al, 2007). (From Yeung CK, Barker GM, Läckgren G. Pathophysiology of bladder dysfunction. In: Gearhart JP, Rink RC, Mouriquand PDE, editors. Pediatric urology. 2nd ed. Philadelphia: Elsevier Saunders; 2010. p. 353–65.) Overactive bladder is characterized by phasic involuntary detrusor contractions, which may occur either spontaneously or on provocation. The exact pathogenesis for detrusor overactivity has not been resolved. Various underlying causes have been proposed: (1) failure of maturation of CNS control mechanisms; (2) low detrusor levels of inhibitory vasoactive intestinal peptide; (3) abnormal enkephalin-mediated inhibitory neurotransmission; (4) denervation hypersensitivity; and (5) alterations in the properties of the detrusor myocytes (Wein and Barrett, 1992; Harrison and Abrams, 1994; Brading, 1997). Detrusor overactivity was frequently found in children with both diurnal and nocturnal enuresis (Bauer et al, 1980; Nevéus et al, 2007). However, the precise relationship between detrusor overactivity contraction exhibited on a cystometrogram and different types of urinary incontinence is still debated (Nevéus et al, 2007). Children with an unstable bladder typically present with a sudden urge to void that can be only be partially suppressed. Often a girl with an unstable bladder will squat down on the heel of one foot, demonstrating the pathognomonic sign of Vincent curtsy. This can be normal, increased (hypersensitive), reduced (diminished), or absent (no sensation).
Normal Bladder Function in Infants and Children
Anatomy of Bladder
Innervation of Bladder
Development of Normal Bladder Function and Micturition Control
Change in Bladder Functional Parameters
Voiding Frequency
Bladder Capacity, Voided Volume, and Emptying Efficiency
Detrusor Pressure at Voiding
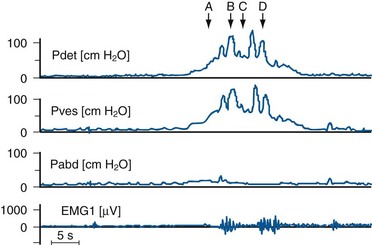
Evolution of Normal Micturition Control
Transitory Detrusor-Sphincter Discoordination in Infancy
Epidemiology and Classification of Non-Neuropathic Bladder-Sphincter Dysfunction in Children
Epidemiology and Prevalence
Categorization of Non-Neuropathic Bladder-Sphincter Dysfunction in Children
Etiologic Classification of Bladder Dysfunction
Derangement of Nervous Control Conditions
Disorders of Detrusor and Sphincteric Muscle Function
Structural Abnormalities
Functional Classification of Bladder Dysfunction
During the Filling (Storage) Phase
Detrusor Activity
Bladder Sensation
Dysfunctional Elimination Syndrome
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Non-Neuropathic Dysfunction of the Lower Urinary Tract in Children
Relationship among Bladder-Sphincter Dysfunction, Vesicoureteral Reflux, and Recurrent Urinary Tract Infections
• The bladder has dual function (storing and emptying) and dual innervation (central somatic and autonomic nervous systems).
• Congenital malformations of the CNS (e.g., myelomeningocele, spina bifida occulta, caudal regression syndrome, tethered cord syndrome)
• Congenital conditions (e.g., muscular dystrophy, neuronal dysplasia, megacolon-megacystis syndrome)
• Congenital conditions (e.g., bladder exstrophy, epispadias, cloacal anomaly, ureteroceles, posterior urethral valves and other urethral anomalies, prune-belly syndrome)
• Functional disorder of filling: overactive bladder, overdistension of bladder or insensate bladder, which may be associated with fecal impaction or rectal distension with infrequent call to stool.
• Functional disorder of emptying: over-recruitment of pelvic floor activity during voiding, causing interrupted and/or incomplete emptying also associated with defecation difficulties due to nonrelaxation of pubo-rectalis, pain on defecation, or even anismus (Bower et al, 2005).