Fig. 5.1
IVU plain films showing stones. Plain full length film from an IVU series showing multiple calculi in the lower pole calyces of the left kidney. Calculi are best demonstrated prior to injection of intravenous contrast media, as the high density contrast can obscure small calculi
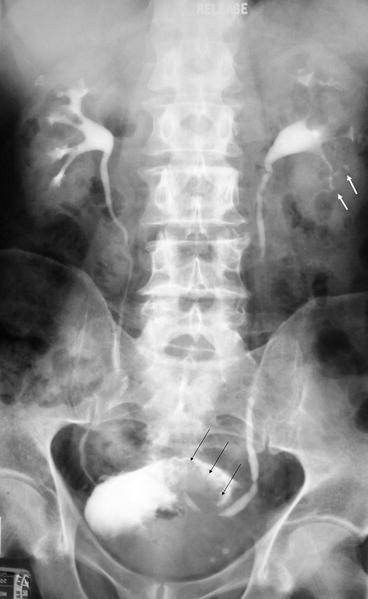
Fig. 5.2
IVU small subtle upper tract TCC lesion. Full length film from an IVU series demonstrating a large filling defect in the bladder, confirmed as a large TCC on cystoscopy (black arrows). Within the lower pole calyces there are small subtle filling defects (white arrows) which were suspected further foci of TCC. This was confirmed on the cystonephroureterectomy specimen
In an emergency setting, low dose unenhanced CT through the renal tract (CT KUB) has replaced IVU in patients with painful haematuria and suspected urinary lithiasis. CT IVU has 3 main applications: (1) evaluating the urinary tract in patients with suspected injury. CT IVU is particularly accurate in assessing severity of renal parenchymal and collecting system injury. Injury to the collecting system is seen as contrast extravasation from the intra-renal collecting system, pelvi-ureteric system and urinary bladder (2) confirming the presence of iatrogenic ureteric/bladder injuries following pelvic surgery. This may be seen either as obstruction of the collecting system in instances where the ureter is occluded or as contrast extravasation when the ureter is wholly or partially transected. (3) in patients with unexplained haematuria in the absence of urinary lithiasis-helps in demonstrating urothelial carcinoma that appear as filling defects either in the intra-renal collecting system, ureters or the urinary bladder.
Patient Preparation for IVU and CT IVU
The patient is starved for at least 4 h prior to the study and is ambulant for 2 h, in order to reduce the amount of bowel gas overlying and obscuring the renal tract. The routine use of purgatives is no longer employed as it does not significantly improve diagnostic quality. Fluid restriction is no longer advocated as dehydration is associated with an increased risk of contrast medium nephrotoxicity. The patient is advised to empty their bladder prior to the examination. Diabetic patients on Metformin no longer need to stop medication but require close monitoring if their blood sugar either by the patient or general practitioner. Pregnant women are advised not to undergo an IVU unless potential benefits outweigh risks to the fetus.
Precautions and Contraindications
Patients with diabetes, multiple myeloma, sickle cell disease and infants are at increased risk of nephrotoxicity and good hydration prior to the examination is advised in these patients. Renal impairment is a relative contraindication for the use of iodinated contrast medium due the risk of precipitating severe renal failure. Poor renal function results in poor renal contrast uptake, concentration and excretion, thereby limiting visualisation of the collecting system. Those with mild renal impairment may be given iso-osmolar non-ionic iodinated contrast medium such as Iodixanol, which is less nephrotoxic than standard non-ionic iodinated contrast agents in this group [2]. Patients with a history of previous severe contrast medium reaction should be excluded (Table 5.1).
Table 5.1
Cautious use of iodinated intravenous contrast medium (ICM)
1. History of previous allergic reaction to ICM: absolute contraindication to use of ICM excluding those with previous mild flushing or nausea |
2. Asthmatics: prophylactic oral steroid cover given prior to the procedure according to local policy and guidelines |
3. Those at risk of nephrotoxicity; |
Renal impairment (can be given to patients with renal failure having regular dialysis when discussed with clinical team) |
Known diabetic nephropathy and those on Metformin (follow local guidelines regarding the use of ICM in patients on Metformin) |
Severely debilitated and dehydrated patients |
IVU Technique
An initial full-length film to include the renal area and bladder is taken to assess technical factors and identify renal calcification, which may become obscured on later contrast enhanced films. A standard adult dose of 50 ml of 350–370 g Iodine/ml (I/ml) or 100 ml of 300gI/ml contrast medium is administered intravenously. This can be altered for patients larger or smaller than the average sized 70 kg adult. A standard sequence of films is obtained at timed intervals, with variations tailored to the individual. The series includes; immediate, 5 and 10-min post contrast renal area films, 15-min full length and post-micturition films. The immediate post contrast film demonstrates renal parenchymal enhancement with contrast medium uptake in the proximal tubules and is termed the nephrographic phase. This enables renal size, position, contour and parenchymal integrity to be evaluated. Excretion of contrast medium into the calyceal system is seen from the 5-min film onwards. Collecting system opacification can be augmented with the use of abdominal compression and a variety of devices are available for this. Compression serves to impede ureteric emptying and enhance pelvicalyceal distension and is applied after the 5-min film if the pelvi-calyceal system is not obstructed. Compression is contraindicated in patients with acute abdominal pain, recent surgery, a known abdominal aortic aneurysm or other large abdominal mass. Compression is released once adequate views of the upper tract have been achieved. This is followed by full-length film to demonstrate contrast medium within the lower ureters.
Diagnostic Value of the IVU
Deformity or calyceal compression or distortion of the renal contour seen on nephrographic phase images indicates a focal parenchymal mass. The nature of the focal mass cannot usually be determined further on IVU and the differential diagnosis includes a simple cyst, renal carcinoma or other benign lesions. In practice, ultrasound or CT are indicated for detection and characterisation of focal renal masses. In the investigation of haematuria, IVU images must be carefully examined for presence of any filling defect within the renal collecting system, ureters or bladder, which may indicate urothelial tumours. These are frequently small and may be seen as a broad based flat or polypoid filling defect arising from the wall. In some cases, slight irregularity of the wall may be the only indication of a tumour (Fig. 5.2). Large tumours may cause obstruction and dilatation of a single or group of calyces (Fig. 5.3). As urothelial carcinomas (UCa) arise on a background of dysplastic urothelium, bilateral or multiple synchronous or metachronous lesions may occur in up to 38 % [3, 4]. The IVU still has a role in excluding multifocal disease in patients with bladder cancer, and in the follow up and surveillance of treated patients.
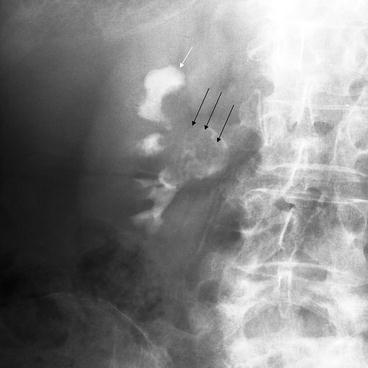

Fig. 5.3
IVU Large lesion with dilated and amputated calyx. A cross-renal image demonstrating a large renal pelvic TCC (black arrows). The lesion invades and obstructs the infundibulum of the upper pole calyx resulting in a dilated, obstructed and amputated upper pole calyx (white arrow)
Alternatives to the IVU
Multi-detector row CT (MDCT) has made a significant impact in all areas of radiology in recent years (see “Computed tomography” section). It enables the fast scanning of a large volume of the patient in a single breath-hold. Thin slices as narrow as 1.25 mm, of the patient can be reconstructed resulting in much improved spatial resolution. These images can be further reconstructed into sagittal and coronal planes. Images of contrast filled structures can be also reconstructed to produce CT angiographic images in 3 dimensional planes. CT has almost entirely replaced the IVU. Plain non-contrast CT KUB has replaced the IVU in the diagnosis and management of stone disease. In the work up of haematuria, when renal tract malignancy is suspected, CT IVU has an increasingly important role. Excretory phase CT can now be used to evaluate the calyces, renal pelvis and ureters and provide images akin to that of a standard IVU [5] (Fig. 5.4).
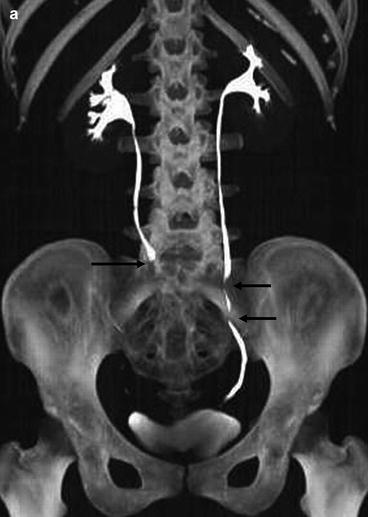
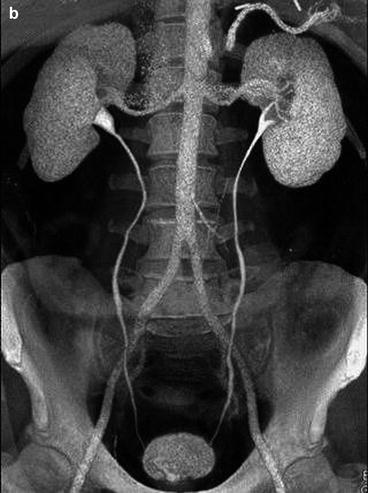


Fig. 5.4
Normal CT IVU. (a) A maximum intensity projection image from a CT IVU series demonstrating normal renal calyces, ureters and bladder. Peristalysis within both ureters can result in under distension, as seen in the distal right ureter or as linear areas of contrast interruption as seen in the left ureter (arrows). (b) Volume rendered 3D image from the same patient demonstrating the detail of the renal parenchyma overlying the calyces the whole length of the ureters and bladder
CT urography is performed with a combination of unenhanced, nephrographic phase and excretory phase imaging. As in standard IVUs, renal tract calcification is detected on unenhanced images and focal renal parenchymal lesions are detected and characterised on corticomedullary (40-s) or nephrographic phase (90-s) imaging. Images obtained 10–15 min after intravenous contrast administration demonstrate contrast filled calyces, ureters and bladder (excretory phase) and are ideal for evaluating the urothelium and filling defects within the collecting systems. The advantage of this technique is that comprehensive evaluation of the renal tract can now be achieved during a single examination [6, 7]. The disadvantages of the technique include cost and time implications as well as radiation dose considerations. The estimated dose equivalent for the CT urogram is approximately 14.8 mSv +/− 3.1 (standard deviation) compared with about 1.5 mSv for a typical standard IVU [8]. However, recent studies using dual phase split bolus protocols, which obtain images in the nephrogenic and delayed phases simultaneously have shown approximately 65 % reduction in radiation exposure without associated reduction in the urinary tract opacification [9]. The guidelines published by the European Society of Urogenital Radiology (ESUR) in 2008 justified the use of CT urography as the first-line imaging investigation for patients with macroscopic haematuria at high-risk for urothelial cancers. They defined high risk as patients above 40 years, macroscopic haematuria, smoking history, history of current or previous GU malignancy and occupational exposure to urothelial carcinogens [10].
Magnetic Resonance (MR) Urography
MR Urography combines the advantage of CT urography in being able to investigate both the renal parenchyma and the urothelium in a single examination. MRI is safe for patients with iodinated contrast allergies or radiation exposure considerations where standard IVU or CT urography are contraindicated e.g. pregnancy. Both dilated and non-dilated renal collecting systems can be visualised using MR urography which is achieved by using either heavily T2-weighted (T2W) sequences or gadolinium-enhanced T1 weighted (T1W) sequences [11]. Heavily T2W MRI sequences generate high signal intensity from simple fluids such as urine, while suppressing signal intensity from surrounding tissues.
Thin section coronal images of the urine filled collecting system provide IVU like images. T2- weighted techniques are fast imaging techniques, which can be successfully applied to patients presenting with painful hydronephrosis of pregnancy in the detection of calculi. In this group of patients both standard and CT IVUs are contraindicated due to the radiation burden and necessary use of iodinated contrast media (Fig. 5.5). Gadolinium-enhanced MR urography relies on contrast excretion in the same way as standard or CT urography to visualise the collecting system. Suboptimal collecting system opacification may limit this technique in the presence of markedly impaired renal function or high-grade urinary obstruction. While the presence and level of ureteric obstruction can be evaluated, the absence of signal from calculi makes them difficult to visualise with both MRI techniques.
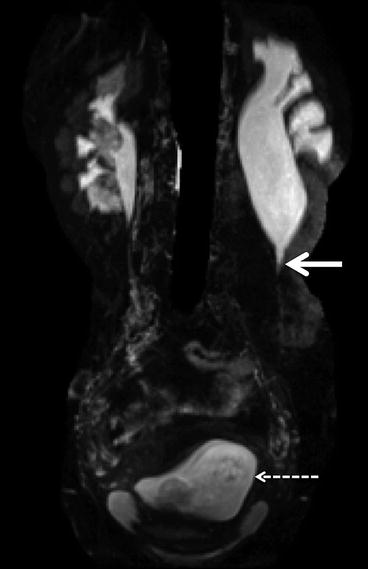

Fig. 5.5
Magnetic resonance urography. Coronal MRU in a pregnant woman presenting with left loin pain and hydronephrosis. The solid arrow demonstrates a typical PUJ obstruction as the cause of the hydronephrosis. The dashed arrow shows the early gravid uterus
Ultrasound
General Principles
Ultrasound imaging is based on the principle that when sound waves are directed into the body by a transducer placed on the skin surface, some will be reflected back to the body surface and detected by the same transducer. The transducer surface is made of material that has the unique property of expanding or contracting when a voltage is applied across it, known as the piezoelectric effect. When the transducer is in contact with skin and a voltage applied, the piezoelectric material expands and compresses an adjacent layer. This pressure induces a corresponding voltage to the next layer of the material, which also expands. The mechanical energy thus created by this wave of compression within the probe is transmitted to the skin surface and propagates through the patient. The propagation and reflection of sound wave through the patient is dependent on density and elasticity of the tissues (acoustic impedance). Reflected echoes returning from the patient to the probe induce a voltage which can be detected by the transducer and converted into a grey scale image. The reflection of sound waves is greatest where there is a large difference in acoustic impedance of two tissues. Thus soft tissue structures reflect more echoes than fluid and appear bright or echogenic. Fluid, which absorbs sound, appears dark, or hypoechoic. The time delay between the initiated and returning pulse to the probe is proportional to the distance the beam has travelled, and spatial information as to the position and depth of tissues are incorporated into the image composition. Constant pulses of sound waves are produced to generate fast real time images of moving body tissues, including flowing blood (Doppler ultrasound). Bone and air reflect sound and cannot be imaged, whereas fluid in simple cysts or bladder does not reflect sound waves, which pass through and are available for imaging deeper structures, a phenomenon termed acoustic enhancement. This is of use in the pelvis where the urine filled bladder is used as an acoustic window for visualising deeper pelvic organs such as the prostate gland and uterus.
Advantages
Ultrasound (US) is a non-invasive real time imaging method, does not use ionising radiation, and is well tolerated by patients. The examination does not require special preparation. It poses no real risk to patients making it ideal for repeated surveillance imaging without the radiation burden associated with CT.
The ability to image flowing blood in real time is a major advantage of ultrasound, which can detect tumour extension into the renal vein and IVC in the case of renal and adrenal carcinomas. The demonstration of vascularity within a lesion can aid its characterisation (Figs. 5.6 and 5.7). The real time aspect of ultrasound is well suited to guide procedures such as fine needle aspiration, biopsy and drain insertions. The echogenic tip of a biopsy needle for example, can be easily demonstrated with ultrasound, providing a clearly visible route for safe insertion. Intraoperative ultrasound probes have also been designed which can be used to localise tumours during a radiofrequency ablation and partial nephrectomy.
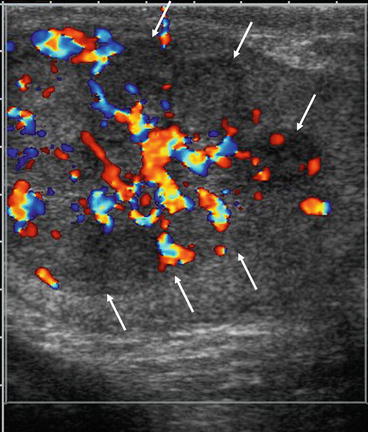
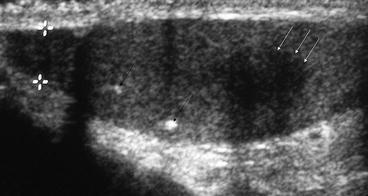

Fig. 5.6
Testicular non seminomatous germ cell tumour. Ultrasound image of a large heterogenous testicular mass (white arrows) replacing the whole testes with increased vascularity. Although the ultrasound features are non-specific, the combination of a focal mass and increased vascularity is high suggestive of a germ cell tumour

Fig. 5.7
Testicular seminoma. Ultrasound image demonstrating a small focal testicular lesion (white arrow) on a background of testicular microlithiasis (black arrows). The lesion is poorly vascular and well defined. The lesion was confirmed as a testicular seminoma
Disadvantages
The success of US is very operator–dependent and images may not always be reproducible between different operators. Although a representative sample of reference images are usually saved onto hardcopy or digital archiving, images are generally best appreciated dynamically during the scan. Comparing a current study with saved hardcopy images from a previous study is not as accurate as it is with CT or MRI.
Patient factors such as body habitus may influence the quality of the images produced with greater attenuation of the ultrasound beam occurring in larger patients, resulting in poor visualisation of deeper structures. As the ultrasound beam is absorbed by air, the presence of prominent bowel gas in the abdomen or pelvis may limit visualisation of deeper organs.
Ultrasound in Genito-Urinary Cancers
There is a wide application of US in genitourinary cancers. Some of these indications are summarised in Table 5.2
Table 5.2
Role of ultrasound in urologic oncology
A. Kidney |
1. Investigation of haematuria |
2. Detection of incidental renal cell carcinoma |
3. Characterisation of cystic renal lesions |
4. Distinguish between adrenal and upper pole renal mass |
5. Ultrasound guided intervention: |
Biopsy of renal masses |
Radiofrequency ablation of renal cell carcinoma |
Intra-operative ultrasound to aid nephron-sparing surgery |
6. Tumour staging |
Perinephric and local invasion |
Venous invasion |
Characterisation of liver lesions |
7. Follow up and surveillance eg. particularly testicular cancer |
8. Assessment of suspected hydronephrosis in patients with pelvic malignancy |
B. Bladder: Investigation of haematuria |
C. Prostate: (Transrectal ultrasound; TRUS): |
TRUS guided biopsy in patients with raised PSA, |
TRUS guided insertion of fiducial markers for image guided radiotherapy radiotherapy (IGRT) |
D. Testes: Investigation of palpable testicular mass |
Surveillance of normal testis after cancer treatment |
Assessment for suitability for partial orchidectomy |
Ultrasound of the urinary tract forms part of the early screening of patients with haematuria, aimed at excluding renal cell carcinoma or tumours of the bladder and renal pelvis.
Renal Masses
Renal cell carcinomas (RCC) are typically very vascular solid lesions on US showing multiple collateral vessels or intra-tumoural arteriovenous shunting on Doppler ultrasonography. The widespread use of CT and ultrasound has contributed to the increased detection of incidental renal carcinomas in recent years. Thus more RCCs are being detected at an early, asymptomatic stage which has contributed to improved survival [12, 13]. Ultrasound is used to diagnose and characterise variety of renal lesions. Simple renal cysts are extremely common and when confidently diagnosed on ultrasound need no further follow up unless they become symptomatic. Ultrasound can be used to characterise complex cystic lesions and highlight those which have features suspicious for malignancy and warrant consideration for surgical excision. The features suggestive of malignancy include the presence of thick wall and septae, internal solid components, vascular flow within the lesion and associated retroperitoneal lymphadenopathy. Doppler ultrasound is highly sensitive for the detection of vascularity, the presence of which in soft tissue components of a cystic mass is suspicious for malignancy. Ultrasound can be used to characterise very small (<1.5 cm) indeterminate renal lesion detected on CT. These masses may represent either solid lesions which will have vascular flow or hyperdense cysts which are hypoechoeic on US and have no internal vascular flow.
The presence of bilateral or multifocal RCCs clearly has an impact on the choice of patient management and careful ultrasound examination of the remainder of the kidney and of the contralateral kidney should be made. This is particularly important in patients with von Hippel Lindau (VHL) syndrome where multiple renal cell carcinomas may exist, often on a background of multicystic kidneys (see Chap. 17). Complex features of any cysts should be carefully evaluated. Renal cell carcinomas arising in small, complex cysts (<1.5 cm) in these patients are typically slow growing and are often followed up for some time.
Ultrasound may be used in conjunction with CT or MRI although ultrasound is less accurate than CT or MRI in RCC staging and perinephric invasion is poorly demonstrated. It is helpful however in diagnosing tumour extension into the renal vein and IVC (Fig. 5.8) which can be diagnosed with up to 75 % sensitivity [14], or higher when clinically important IVC thrombus is considered [15]. Supra-diaphragmatic extension is not well seen and if suspected, echocardiography should help to exclude tumour extension into the right atrium.
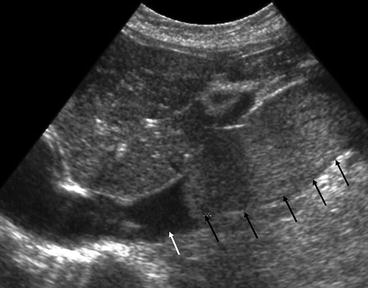

Fig. 5.8
Inferior vena cava thrombus on US. Longitudinal section of the abdominal IVC. The normal IVC is seen as a fluid filled tubular structure (white arrow) on ultrasound. A large tumour thrombus is seen as a hyperechoic lesion within the lumen expanding the IVC (black arrows). The thrombus is limited to the infra-diaphragmatic IVC with its superior extent clearly demonstrated on US
Urothelial Tumours
Ultrasound is supplemented by IVU in the work up of patients with haematuria. Alternatively for the detection of renal and urothelial lesions, a CT IVU would replace both studies. CT IVU has the added advantage of staging a detected malignancy. The renal pelvis, proximal ureter and bladder can usually be visualised with ultrasound. The ureter however, unless dilated, is not well visualised throughout its length. The bladder should be well filled for its optimal examination with ultrasound as focal areas of wall thickening, representing plaque lesions of urothelial carcinoma can be mimicked by a collapsed bladder wall.
In patients with haematuria, demonstration of an echogenic mass with vascular flow on ultrasound within the renal pelvis, proximal ureter or bladder suggests the presence of malignancy, commonly TCC. Visualisation of the upper tract collecting system is improved in the presence of obstruction where an echogenic mass may be seen outlined by hypoechoic urine. Differentiation of echogenic debris from tumour within the collecting system or bladder can be made by the detection of vascularity within tumours on Doppler scan. Doppler can therefore aid characterise of an extrinsic mass or collecting system filling defect demonstrated on IVU and US. US may demonstrate evidence of extension of tumour outside of the renal collecting system or bladder, but overall staging is achieved with a combination of cystoscopy, CT and MRI.
Adrenal Glands
Normal adult adrenal glands are not usually visualised with ultrasound given their small size and deep location. Incidental adrenal mass lesions may occasionally be detected with ultrasound. Ultrasound may be useful to interrogate a large suprarenal mass seen on axial CT, to determine its origin, exclude invasion of the liver, kidney, tail of pancreas or IVC and renal vessels. CT and MRI are the investigations of choice for the characterisation of adrenal masses and staging of adrenal carcinoma. Large adrenal masses may be biopsied under US control but only after biochemical exclusion of pheochromocytoma.
Testis
Given its superficial location, the testis is well visualised with ultrasound making it the first line investigation of a patient with a palpable scrotal mass. Benign lesions of the epididymis such as simple cysts are common and when a lesion has been detected clinically, ultrasound is used to determine whether it lies within the testis or not. Benign solid lesions within the testis are extremely rare (dermoids and epidermoids), and all such lesions, in the absence of clinical features to suggest infection, are presumed to be malignant. testicular germ cell tumours are typically solid, heterogeneous masses which may be multiple, and are often highly vascular (Figs. 5.6, 5.9 and 5.10). Careful ultrasound examination of the contralateral testis is essential.
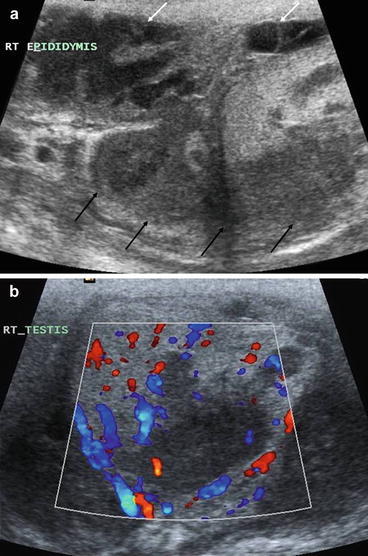
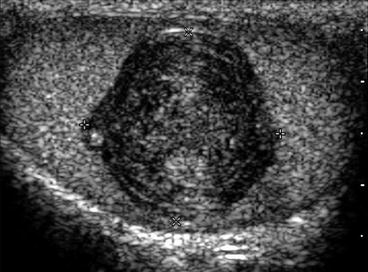

Fig. 5.9
Scrotal TB. (a) Longitudinal image of the epidydimis which is thickened and hypoechoeic (black arrows) with extensive surrounding inflammatory change. There is loculated fibrinous free fluid in the scrotum (white arrows) indicating an inflammatory process. (b) Transverse image of the testes, demonstrating focal areas of hypoecoic change in the testes with an associated increase in vascularity of the whole testes. These features are highly indicative of an epididimo-orchitis

Fig. 5.10
histologically confirmed testicular epidermoid cyst. A longitudinal image of the testes showing a well defined smooth mass with internal laminated rings. These echogenic rings are highly suggestive of an epidermoid cyst
If such a suspicious testicular tumour is detected with ultrasound urgent urology referral should be made. Staging of testicular tumours is primarily centred on the detection of retroperitoneal lymphadenopathy and excluding distant metastases to the lungs, bone or brain. CT is the imaging modality of choice for staging (see CT staging).
Prostate
Prostate ultrasound is indicated in patients with elevated prostatic specific antigen (PSA) in whom prostate carcinoma is suspected. Locally advanced tumours may be visualised on ultrasound within the bladder, and hydronephrosis due to outflow obstruction by a large or invasive tumour can also be diagnosed.
Endocavity ultrasound with a transrectal transducer (TRUS) places the beam in close proximity to the prostate gland. This is performed with the empty bladder. Anatomical detail of the prostate gland can be appreciated with clear delineation between the central and peripheral zones. Tumours are demonstrated as well defined hypoechoic nodules within the peripheral zone with increased vascularity on colour flow Doppler (Fig. 5.11). Carcinomas may also be ill defined and isoechoic or hyperechoeic to the normal prostate gland. Carcinomas in the central gland cannot be distinguished from benign adenomatous changes of the central zone. Ultrasound guided targeted and non-targeted biopsy of multiple sites of the peripheral zone is the main indication for TRUS, in those suspected of prostate carcinoma. Local staging is best achieved with MRI in those considered for radical treatment and with CT for advanced carcinomas to identify nodal disease and distant metastases. This is described in detail later..
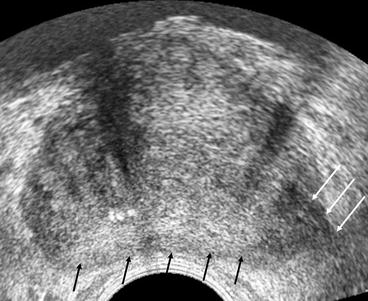

Fig. 5.11
Transrectal ultrasound of the prostate. Transverse section through the mid gland of the prostate. The peripheral gland is seen a homogenous hyperechoic area (black arrows). A focal carcinoma is noted within the left peripheral zone as a hypoechoeic area (white arrows). This extends beyond the prostatic margins into the peri-prostatic fat indicating extra-capsular disease
Contrast Enhanced Ultrasound
In recent years intravascular contrast agents have been developed which when administered enhance both grey scale and Doppler ultrasound [16, 17]. The ultrasound contrast agents, which have been developed consist of small (<7 μm), encapsulated microbubbles. They are small enough to pass through the pulmonary and capillary circulation and stable enough to withstand hydrostatic pressure within the vascular system and acoustic pressure from the ultrasound wave. When administered they remain in the vascular system. Specific properties of the microbubble capsule and gas within induce greater reflection of the acoustic wave, resulting in increased backscatter. This increases the echogenicity and enhances the grey scale or Doppler image. New ultrasound imaging parameters have been developed which increase the conspicuity of microbubble enhanced backscatter.
The images produced using US contrast agents are greatly enhanced compared to standard grey scale and Doppler imaging and may aid in the detection and characterisation of small isoechoic renal or prostatic malignacies.
Computed Tomography
General Principles
The production of an image by Computed Tomography (CT) is based on differential absorption of an x-ray beam by tissues within the body in the same way as conventional radiography. The amount of absorbed x-rays depends on tissue density, with dense tissue absorbing greater number of x-rays and thereby appearing whiter than less dense tissue which absorb fewer x-rays.
In CT the x-ray beam is collimated into a narrow beam, which passes through a thin slice of the patient. The attenuated x-ray beam, emerging from the patient, is absorbed by detectors, which are capable of differentiating very subtle differences in tissue density. CT therefore has a much greater contrast resolution than plain x-rays and eliminates problems of superimposition of overlying structures to a much greater extent. The information collected by the detectors is converted into an arbitrary scale (Hounsfield units; HU) based on the attenuation of the x-ray beam by the tissues it has passed through, which varies with differing densities of body tissues. Bone or calcification are the most attenuating and are given a value of +1000HU while air, the least attenuating is given a value of −1000HU. The values are converted to a grey scale image and assigned a brightness level with the highest numbers white and the lowest numbers black. The range (window width) and mean value (window level) of density units is selected to optimise visualisation of different tissue densities of interest. Tissues of densities outside of the range selected will not be discernable and be either totally black or totally white. Standard settings can be selected to display lung, bone or soft tissue ‘windows’ as required.
Types of CT
Spiral CT
Newer generation scanners utilise a method of volume rather than slice by slice acquisition. A continuous fan x-ray beam, rotating around the patient, traces a spiral path as the patient is moved through the gantry of the machine. Data is continuously acquired through each 360° rotation. Thus a volume of tissue per rotation rather than a slice is imaged. The distance the patient is moved through one revolution of the tube is equal to the slice thickness. Decreased scan acquisition time is a significant advantage of this technique enabling imaging of a larger volume of the patient in a singe breath-hold. This eliminates problems with variation of respiration with each slice. Partial volume effects are also minimized.
Multi-detector CT (MDCT)
It is now possible to acquire data from more than slice thickness simultaneously using parallel banks of detectors. Spiral scanners are now available which are able to acquire up to 128 slice thicknesses in one tube rotation. Data is thus acquired much faster than with a single slice or spiral scanner.
Much thinner slices can be acquired resulting in greatly improved spatial resolution and reduced partial volume effects. In addition, post processing of the large volume of thin slices acquired enables 3 dimensional (3D) and multiplanar image reconstruction. 3D reconstruction applications include CT angiography, virtual endoscopy and CT ‘fluoroscopy’. Multiplanar images enable the tumour and its relationship to surrounding structures to be delineated accurately. Combined with CT renal angiography it plays an important role in preoperative surgical planning for renal cell carcinoma, particularly when nephron-sparing surgery is being considered (Fig. 5.12).
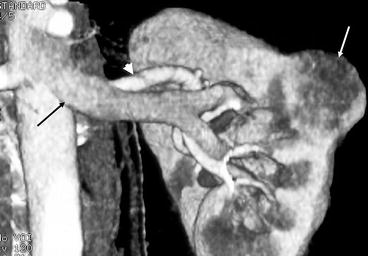

Fig. 5.12
3D volume rendered multi-detector CT reconstruction. A coronal 3D reconstruction of the left kidney showing a focal small renal carcinoma (white arrow). Its location in the kidney is well demonstrated along with its relation to the central renal vessels. The main renal artery is shown by the black arrow and the main renal vein by the arrow head. Both have a normal hilar configuration
Patient Preparation and Technique
Oral contrast medium or water is administered prior to the examination to optimise opacification of small and large bowel to allow better anatomical detail, particularly in the pelvis or retroperitoneum, where the presence of unopacified loops of bowel can be misinterpreted as soft tissue masses or lymph nodes. For bladder and prostate tumours a moderately full bladder is preferable.
For characterising space occupying lesions of the kidney or an adrenal gland, initial unenhanced scans are obtained through the renal or adrenal area at 5 mm slice thickness. The scan is obtained in a single breath-hold at either maximum inspiration or expiration. This allows visualisation of calcification within the lesion and enables the density of the lesion to be calculated. The scan is then repeated following administration of intravenous contrast medium unless contraindicated (see Table 5.1). 100 ml of non-ionic iodinated contrast medium (300–350 mg iodine/ml) is administered via a pump injector at a rate of 3–5 ml/ s. To optimise characterisation of liver, renal or adrenal lesions scans are obtained at variable times to maximise vascular enhancement. For renal lesions scans are obtained at 40 s and 90 s following contrast infusion. For adrenal lesions scans are obtained at 60 s and 15 min following contrast administration and for liver lesions, scans are acquired at 30 s, 70 s and delayed scans up to 15 min may be required.
In the kidney 40 and 90 s equate to corticomedullary and nephrographic phases of enhancement respectively. While the nephrographic phase is more sensitive for the detection and characterisation of small renal lesions, evaluation of the kidneys during both phases provides optimum information. Images obtained during the corticomedullary phase detect low grade enhancement in papillary carcinomas and allow for evaluation of the renal vein and identification of accessory renal arteries. Images obtained at 3–15 min post contrast administration demonstrate contrast within the pelvicalyceal system (excretory phase), and can be used to delineate tumour within the renal pelvis or ureter. In centrally located RCCs, the proximity of the lesion to the pelvi-calyceal system and the hilar vessels allows evaluation for the suitability for nephron sparing surgery (NSS) (Fig. 5.13).
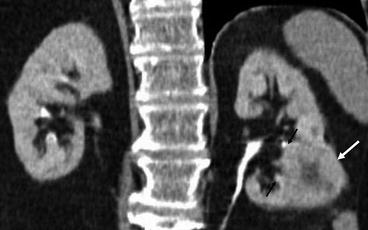

Fig. 5.13
Coronal multi-detector CT reconstruction. A CT IVU coronal reconstructed image. A left lower pole renal cell carcinoma is demonstrated (white arrow). The medial margins of the tumour extend up to the renal sinus and abuts the calyces (black arrows)
For a full staging scan, axial 2–5 mm images are acquired through the whole abdomen from the level of the diaphragm to the pelvis. These are obtained with the 90 s scan when a renal protocol scan has been performed. Alternatively, scanning is timed to commence at approximately 60 s following contrast infusion so as to image the liver during maximum portal venous phase enhancement, the optimum time for the detection of most hepatic metastases. For tumours such as renal cell carcinoma and testicular carcinoma which have a propensity to metastases to the lung, images through the thorax are initially acquired at approximately 20–40 s after contrast infusion.
The Role of CT in Urologic Oncology
There are a myriad of uses for CT in urological cancer management. These are summarized in Table 5.3.
Table 5.3
Role of CT in Genito-Urinary cancers
A. Kidney |
1. Detection of incidental renal cell carcinomas |
2. Characterisation of cystic renal mass lesions |
3. Tumour staging |
Local: perinephric and organ invasion |
Nodal staging |
Venous invasion |
Distant metastases: lung, liver, bones, brain |
4. 3D CT for surgical planning |
5. Follow up and surveillance |
B. Adrenal |
1. Detection and characterisation of incidental adrenal mass lesions |
2. Primary adrenal carcinoma staging: Local and Distant metastases: lungs, liver, bones |
3. Planning surgical resection in large adrenal masses |
C. Bladder |
1. Tumour staging: Locally advanced disease; Nodal: Iliac and para-aortic lymph nodes |
Distant metastases: lung, brain, liver, bones |
2. Radiotherapy planning |
3. Post chemotherapy evaluation and surveillance |
D. Prostate |
1. Tumour staging: Locally advanced disease; Nodal: pelvic side wall and retroperitoneal |
2. Rising PSA following radical therapy for nodal or bony metastases |
3. Radiotherapy planning |
E. Testis |
1. Tumour staging, Nodal staging and Distant metastases: lungs, brain, liver and bones |
2. Surveillance |
3. Planning retroperitoneal lymph node dissection |
Lesion Detection and Diagnosis
CT does not usually form part of the initial diagnosis of bladder, prostate or testicular carcinomas although incidental detection of a soft tissue filling defect within the contrast filled renal collecting system or bladder may suggest the presence of a transitional cell carcinoma. Contrast enhancement of such a mass confirms the diagnosis and excludes the presence of debris or blood clot.
Occasionally testicular carcinoma might present as large retroperitoneal lymph nodal mass and in a young male patient the possibility of testicular germ cell tumour should be considered. CT has an established role in the detection and characterization of indeterminate renal and adrenal lesions.
Renal Space Occupying Lesions
RCCs are suspected on CT in the presence of a wholly or partially solid, enhancing and often heterogeneous parenchymal renal mass, frequently detected incidentally [12, 13, 18]. Cystic renal cell carcinomas are well recognised and need to be differentiated from complicated benign cysts such as simple cysts which have become infected or bled.
Bosniak Grading of Cystic Renal Lesions
A system of grading the appearances of cystic renal masses with CT, according to the presence of features associated with malignancy has been devised by Bosniak [19, 20], is summarized in Table 5.4. Simple cysts with no suspicious features are within category I and those with increasingly complex features are graded up to category IV, which are frankly malignant. Suspicious features include the presence of thick punctate calcification, wall or septal thickening and the presence of enhancing soft tissue components. Category III and IV lesions have malignant features and should be surgically removed (Figs. 5.14 and 5.15). In category III and IV, 60 % of the lesions are malignant and the main benign lesions are inflammatory and infective diseases. Category II lesions have some complex features such as fine, linear calcification, thin septa of are of increased density, but no enhancing soft tissue. These lesions do not need to be followed up. More recently category IIF has been introduced and includes benign complicated cysts which require follow up over time to confirm stability. Features in this group include those with numerous but thin septa, septal or wall enhancement but no soft tissue component, hyperdense category II lesions which are totally intrarenal or greater than 3 cm [21] also fall into this category (Fig. 5.16).
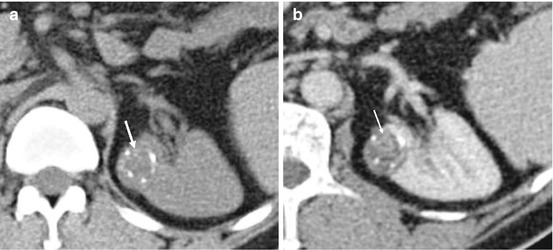
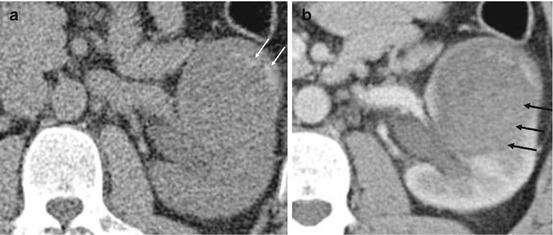




Table 5.4
Bosniak classification of cystic renal lesions
Category | Description |
|---|---|
I | Simple benign cyst with an imperceptible or hairline thin wall that does not contain septa, calcification or solid components. It measures as water density and does not enhance following contrast administration |
II | Benign cyst that may contain a few hairline thin septa. Fine calcification may be present in the wall or septa. |
Uniformly high attenuation lesion of <3 cm that is sharply marginated and does not enhance | |
IIF | Cyst might contain more hairline thin septa. Minimal enhancement of septa or wall. May be minimal thickening of the wall/septa. |
Cyst might contain calcification that may be nodular and thick but does not enhance. | |
Does not contain enhancing soft tissue elements. | |
Non enhancing high attenuation lesions >3 cm | |
III | Indeterminate cystic masses that have thickened irregular wall or septa in which enhancement can be seen. |
IV | Cystic lesions are likely malignant that contain enhancing soft tissue elements. |

Fig. 5.14
Bosniak 3 renal cyst. (a) Pre contrast CT demonstrating a cortical lesion with dense amorphous calcification (white arrow). (b) Post contrast CT showing small areas of soft tissue enhancement (white arrow) within the lesion in keeping with a Bosniak 3 cyst. The lesion was confirmed as a clear cell renal cell carcinoma on histology

Fig. 5.15
Bosniak 4 renal cyst. (a) Pre contrast CT demonstrating a large left renal mass with irregular areas of calcification and low attenuation cystic background (white arrow). (b) Post contrast CT showing diffuse and homogenous enhancement centrally within the lesion (arrows). In view of the size and the enhancement, the lesion is consistent with a Bosniak 4 cyst. The lesion was a papillary carcinoma on histology
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







