Infravesical: Urothelial cancer of the bladder, prostate cancer, urethral carcinoma, carcinoma cervix/uterus/ovary and rectum
Supravesical: Urothelial cancer of ureter and renal pelvis, bladder, primary or secondary Retroperitoneal lymph node involvement, malignant retroperitoneal fibrosis
1.
Obstructive Uropathy: Obstruction to the urinary flow could occur at various levels of upper urinary tract; it could be bilateral or unilateral, supravesical (above vesico-ureteric junction) or infravesical (below vesico-ureteric junction) (Table 9.1).
2.
Renal cell carcinoma (RCC) in solitary kidney
3.
Pre-existing renal disease (Acquired cystic disease in patients who are on dialysis)
4.
Nephrotoxicity induced by high-dose chemotherapy
5.
Malignancies in Renal transplant patients
6.
Other systemic diseases, which affect renal function like diabetes and hypertension
7.
Effects of previous cancer treatment: Radiotherapy, chemotherapy
8.
Renal damage as a result of nephrotoxicity due to analgesics and antibiotics
9.
Paraneoplastic syndromes
Patient Assessment
When assessing such patients there are a few key questions which are essential in the overall decision-making process:
What is the likely course and prognosis of the disease process?
Are there co-morbid conditions which are likely to be more serious than the cancer process itself?
What is the overall level of renal function? What is the differential renal function?
Has the patient got one kidney only? How much function is likely to be lost following surgery? (Partial or total loss)
Is the potential loss of function likely to render the patient dialysis-dependent.
If so, is it short term or long-term.
What would the prognosis of the patient be without treatment compared to dialysis.
Is further treatment (e.g. Chemotherapy) likely to cause deterioration in the renal function.
In acute cases of bilateral obstruction, relief of obstruction by drainage or in selected cases dialysis followed by resuscitation and correcting fluid balance and metabolic acidosis is necessary prior to proper staging and deciding about the definitive management of the malignancy (Chap. 7).
1.
Overall course and prognosis of the disease process.
This is most crucial question in planning the treatment as the clinicians try to work out how far they can push the boundaries of management.
2.
Co–morbid conditions, which are likely to be more serious than the cancer process itself: The patient’s comorbidity such as cardiovascular status, previous history of stroke will influence the prognosis of renal failure management.
3.
Overall level of renal function
Methods of assessing renal function: Assessing the baseline renal function can be done in numerous ways. This basic information is required and forms the basis of the rest of the assessment.
Blood Tests
The simplest is measuring the serum concentrations of urea and creatinine. Blood urea is not generally felt to be a marker of renal function as urea clearance is 50–60 % of GFR [1]. Urea levels are influenced by age of the patient, dietary protein intake and protein metabolism. Similarly blood urea nitrogen (BUN), a product urea has similar limitations.
Serum creatinine (SCr) levels are more reliable than urea and reflect not only renal excretion, but also the generation, intake, and metabolism of creatine and phosphocreatine [2]. Factors other than renal function also influence SCr which include age, weight, nutritional status, gender and ethnicity. SCr levels may not rise above the normal range until the kidney function has become significantly impaired [3]. For example, an elderly malnourished patient may have SCr within the normal range but may well have a markedly impaired glomerular filtration rate (GFR). Conversely a young muscular male may have a SCr that is above the normal range but have a normal GFR. In most instances therefore the SCr is sufficient to assess renal function but is important to remember that serum creatinine levels are also influenced by extrarenal factors.
Creatinine Clearance
As creatinine is not metabolized in the kidney and freely filtered by the glomeruli, it is a simple way of measuring GFR. The urinary creatinine (U) excretion over 24 h when divided by the SCr (P) will provide a measured creatinine clearance ([U] × [urine volume]/[P]). However, as creatinine is also secreted by proximal tubule, the creatinine clearance usually exceeds GFR [4]. This test was used frequently in clinical practice but is subject to error in that it is highly dependent on an accurate 24 h urine collection and the creatinine levels.
Glomerular Filtration Rate (GFR)
Although GFR gives fairly accurate assessment of functioning nephrons it is not frequently used in clinical practice [5]. It can be estimated using several validated formulae. The Cockcroft-Gault formula [6] requires the patient’s weight, age and serum Creatinine (μmol/L). There are likely to be errors in measurements and it is difficult to ensure the quality of the variables [5].
Cockcroft-Gault Formula
![$$ \begin{array}{c}\mathrm{G}\mathrm{F}\mathrm{R}=K\times \mathrm{weight}\times \left[140-\mathrm{age}\right]/\mathrm{Serum}\;\mathrm{Creatinine}\\ {}\left( WhereK=1.23for\ males\ and\ 1.03\ for\ females\right)\end{array} $$](/wp-content/uploads/2016/07/A83119_2_En_9_Chapter_Equa.gif)
MDRD Formula
![$$ \begin{array}{c}\mathrm{G}\mathrm{F}\mathrm{R}=186\times \left[\mathrm{Serum}\;\mathrm{Creatinine}\right]-1.154\times \left[\mathrm{age}\right]\\ {}-0.203\times \left[0.742\;\mathrm{if}\;\mathrm{female}\right]\times \left[1.212\;\mathrm{if}\;\mathrm{black}\right]\end{array} $$](/wp-content/uploads/2016/07/A83119_2_En_9_Chapter_Equb.gif)
Radioisotopes/Radiocontrast Studies
(Chapter 6)– These techniques are the most accurate methods of measuring the GFR in clinical practice [8]. The success of these methods relies on the use of radiopharmaceticals that are freely filtered by the glomerulus and are neither reabsorbed nor secreted by the tubules. The principles of isotope renography are discussed in Chap. 5. These methods however are invasive and expensive and may not be available in all clinical settings. In a study of 122 cancer patients eGFR measured by the equations above was compared with Tc99m DTPA clearance and the data suggested that there were significant limitations to using eGFR compared [8].
How Much Function Is Likely to Be Lost Following Surgery/Treatment?
In order to estimate the potential loss of function post operatively, it is clearly important to know the contribution each kidney makes to the total GFR. As mentioned earlier, this can be assessed using isotope renography. These techniques employ radio-pharmaceutical agent which are either glomerular filtration agents (diethylenetriaminepentaacetic acid -DTPA) or tubular secretion agents (mercaptoacetyltriglycine-MAG3, dimercaptosuccinic acid- DMSA). Following intravenous administration, it is possible to measure renal blood flow and renal cortical function by the isotope activity which is then computed using scintigraphic techniques [9].
By using this information combined with the knowledge of the overall GFR, the single kidney GFR can be determined. As an example, if a nephrectomy is being planned and the contribution of the problem kidney is 10 % for someone with an overall GFR of 80 ml/min (i.e. problem kidney GFR = 8 ml/min) then there should be more than sufficient residual renal function post operatively. If however the affected kidney contributes 50 % or more to overall function in someone with a total GFR of 20 ml/min, the planned nephrectomy may render the patient dialysis dependent. The impact on renal function following nephron sparing surgery however is more difficult to predict but at least patients can be counselled regarding the “worst case scenario”.
The information regarding the degree of residual renal function post surgery is extremely important as it enables clinicians to advise the patients on the potential need for dialysis post operatively either on a temporary or permanent basis. This will be discussed in more detail below.
Is the Potential Loss of Function Likely to Render the Patient Dialysis Dependent?
The threshold GFR at which renal support is required varies according the individual patient and also accepted practice in different renal units. Most though would agree that a GFR of 10 ml/min/1.73 m2 or less is an indication for dialysis (National Kidney Foundation Kidney Disease Outcomes Quality Initiative- NKF-DOQI Clinical practice guideline) [10]. Based on the measurements discussed above one may be able to predict, with some degree of certainty, the likely need for dialysis support post operatively. This information should be relayed to the patient as it may inform their choice of treatment options. As mentioned above, in the case of nephron sparing surgery (or partial nephrectomy) it is more difficult to predict the impact of surgery on renal function.
There are several post-operative scenarios for renal support in GU cancer patients with compromised renal function (Fig. 9.1):
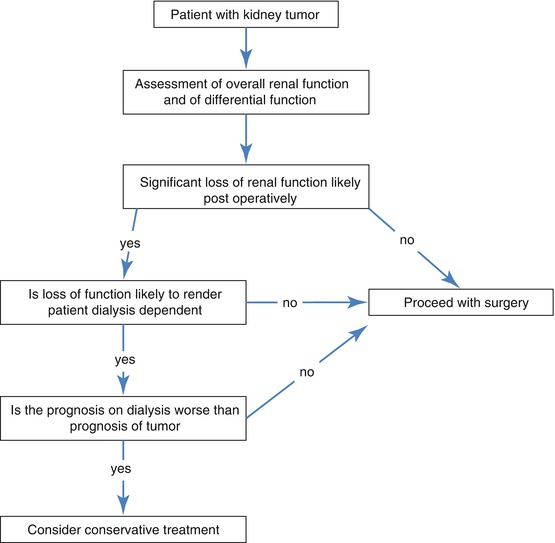

Fig. 9.1
Treatment decision flowchart
1.
Patients with sufficient residual function who do not require postoperative dialysis. Their remaining nephron mass is sufficient for them not to need renal replacement therapy.
2.
Patients who need dialysis in the short term but then become dialysis independent. In this scenario the patient is left with sufficient nephron mass not to require long-term dialysis but the operative procedure has caused acute tubular necrosis and temporary renal shut down. This usually recovers in time but may take up to 6 weeks.
3.
Patient regains independent renal function post–operatively but over subsequent months/years develops remnant nephropathy and end stage renal failure. Once a critical nephron mass is lost a maladaptive response known as hyperfiltration ensues irrespective of original pathology [11, 12]. In simple terms the remaining glomeruli of the kidney(s) hypertrophy. In the short term this increases the GFR but in the long term leads to focal glomerulosclerosis and remnant nephropathy and ultimately to end stage renal failure.
4.
Patient will need dialysis thenceforth. Sufficient renal mass has been removed such that the patient does not have independent kidney function and will thus require long term/permanent dialysis.
Based on the results garnered from the investigations outlined above (overall renal function and split renal function) it is possible, with a modest degree of certainty, to predict which of these scenarios is likely. This information should be passed on to the patient. It also extremely important for the physicians and surgeons to be aware of this as it may influence the decisions regarding surgery. This is especially true if the likely prognosis on dialysis is poor in which case surgery may not be in the patient’s best interest.
Prognosis of Tumour vs. Dialysis
When deciding treatment options it is important to bear in mind the prognosis of the individual patient on long term dialysis if that is a likely scenario. The prognosis on dialysis of a young dialysis patient with no co-morbidity is very different from an elderly patient with serious comorbidities such as heart disease and diabetes in relation to survival. These need to be borne in mind in that the prognosis of the underlying tumour may be significantly better with conservative management in certain individuals.
It is worth remembering that for a debilitated and dependant patient, quality of life on dialysis may be very poor often with multiple admissions to the hospital for initiation and maintenance of dialysis. These facts may also influence treatment decisions (Fig. 9.1).
Perioperative Management
The preoperative management of patient undergoing radical nephrectomy with a normally functioning contralateral kidney does not need any specific measures. Special precautions are however necessary in patients undergoing nephron-saving surgery. Fluid balance is the mainstay of maintaining renal function over the perioperative period.
Preoperative Management
Patients should be volume replete at the time of surgery, which often means they will require a continuous intravenous infusion during the ‘nil by mouth’ period. Although there are little data on preoperative fluid regimens, studies examining hydration prior to contrast examinations have shown crystalloid infusions (at 1 ml/kg/h for 12 h) to be very effective in preventing subsequent renal dysfunction [13]. This however must not be applied to patients who are anuric (i.e. on dialysis) as they run the danger of becoming volume overloaded.
During nephron-saving surgery temporary occlusion of renal artery is required to achieve a bloodless field. To prevent ischaemic injury to the kidney the patient should be well hydrated and intravenous mannitol is given 5–10 min prior to renal artery occlusion to prevent intracellular oedema [14]. Mannitol could be repeated after removal of the vascular clamp to induce diuresis. Cooling the kidney with ice slush to decrease the core temperature to 15–20 °C allows the surgeon with 2–3 h of “safe ischaemia” [15].
Postoperative Fluid Management
The principles of post operative fluid management very much depend on the patients’ urine output and other fluid losses from drains and nasogastric tubes. Other important parameters include daily weights, blood pressure, pulse rate, central venous pressure and daily blood chemistry in the initial post operative phase. Central venous pressure (CVP) measurements should not replace the simple clinical parameters listed above.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







