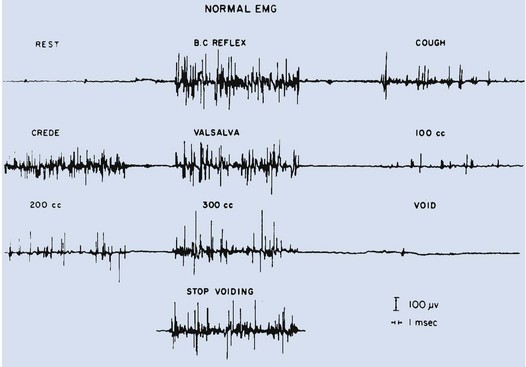
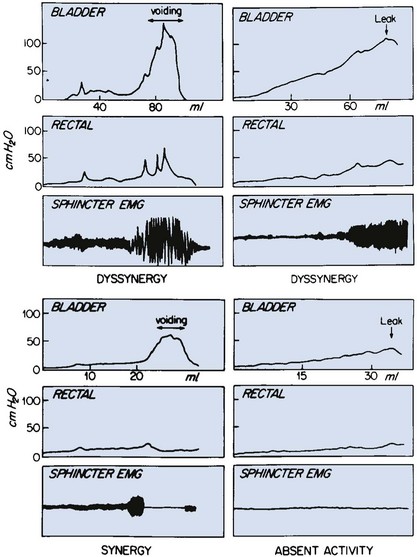
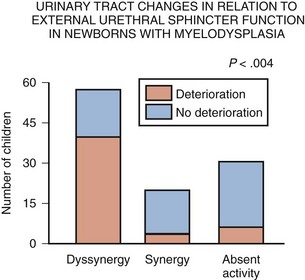
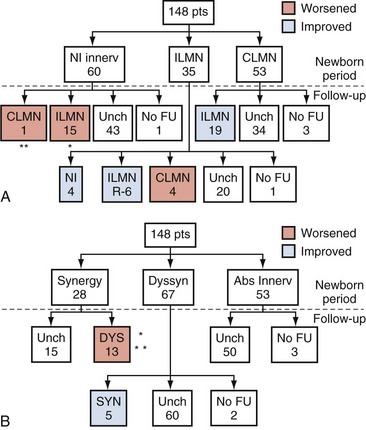
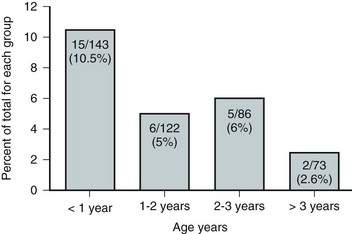
Dawn Lee MacLellan, MD, FRCSC, Stuart B. Bauer, MD Many of the clinical problems seen in pediatric urology are the result of neurologic lesions that affect lower urinary tract function. As pediatric urology developed in the latter half of the 20th century, urinary diversion initially was the mainstay of treatment for these children with either intractable incontinence or normal or abnormal upper urinary tracts (Smith, 1972). The advent of clean intermittent catheterization (CIC) in the early 1970s by Lapides and associates (1972), refinements in techniques of urodynamic studies in children (Gierup and Ericsson, 1970; Blaivas et al, 1977; Blaivas, 1979), and the development of surgical modalities in conjunction with CIC to manage incontinence dramatically changed the way this group of children was traditionally managed. Most pediatric urologic centers consider functional assessment of the lower urinary tract as an integral element in the evaluation process and almost if not as important as radiographic visualization in characterizing and managing these abnormal conditions (McGuire et al, 1981; Bauer et al, 1984). The natural outcome of early functional investigation has been the advocacy of proactive or early aggressive management in the children who are at risk for urinary tract deterioration based on specific hostile urodynamic parameters (Perez et al, 1992; Edelstein et al, 1995; Kaufman et al, 1996). This has resulted in (1) a statistically significant decrease in upper urinary tract deterioration, compared with those children followed expectantly in the past, and (2) a dramatic reduction in the need for augmentation cystoplasty (Wu et al, 1997; Kaefer et al, 1999; Bauer, 2000). Furthermore, there is now evidence that molecular changes occur intracellularly when prophylactic treatment is begun as early as possible (Park et al, 1999; Ohnishi et al, 2000). Because urodynamic testing has become such an integral part of any discussion of the subject, the testing process as it applies to children is discussed first and then various neurologic abnormalities that are prevalent in children are elaborated upon. For purposes of minimizing ambiguity the terminology used in this chapter is based on the International Continence Society (ICS) standardization of terminology documents (Abrams et al, 2002, 2003; Neveus, 2006). Next, the nurse reviews the test and shows the child all the equipment in an attempt to make the patient feel as comfortable as possible. The child is catheterized with either a No. 7 Fr or a No. 11 Fr triple-lumen urodynamic catheter (Cook Urological, Inc, Spencer, IN)—the smaller, the better—after a small amount of liquid lidocaine (Xylocaine) (1%) has been injected into the urethra and held in place for a moment or two. First, the intravesicular pressure is recorded. Then the bladder is drained and the residual urine is carefully measured, yielding a pressure at residual volume (Kaefer et al, 1997). Sometimes it is necessary to aspirate the catheter to get the accurate volume of residual urine, especially if the bladder is underactive (particularly if the child is taking anticholinergic medication) or has been previously augmented and secretes mucus (because it may not drain completely after insertion of a catheter). A small balloon catheter is passed into the rectum at this time to measure intra-abdominal pressure during the cystometrogram to identify artifacts of motion and monitor increases in abdominal pressure during the filling and emptying phases of the study (Bates et al, 1970; Bauer, 1979). Before the bladder is filled, a urethral pressure profile is sometimes obtained by infusing saline through the side-hole channel at a rate of 2 mL/min as the catheter is withdrawn at a rate of 2 mm/sec (Yalla et al, 1980). Many centers have eliminated urethral pressure profile measurements in favor of monitoring detrusor leak point pressure (the subtracted detrusor pressure at which the child leaks when the bladder is filled to its functional or maximal capacity). Abdominal leak point pressure is the abdominal pressure at which the child leaks when straining or coughing but that is not as accurate as the detrusor leak point pressure, which is a proportional reflection of outlet resistance (McGuire et al, 1996). External urethral sphincter EMG is performed using a 24-gauge concentric needle electrode (Diokno et al, 1974; Blaivas et al, 1977b) inserted perineally in boys or paraurethrally in girls and advanced into the skeletal muscle component of the sphincter until individual motor unit action potentials are seen or heard on a standard EMG recorder. Alternatively, perineal electrodes (Maizels and Firlit, 1979) or abdominal patch electrodes (Koff and Kass, 1982) have been used to record the bioelectric activity in the sphincter muscle. Disagreements exist concerning the accuracy of these surface electrode measurements, compared with those obtained with needle electrodes, particularly during voiding. The intactness of sacral spinal cord function is easily measured with needle electrodes by (1) looking at the characteristic waveform of individual motor unit action potentials when the patient is relaxed and the bladder is empty, (2) performing and recording the responses to bulbocavernosus and anal stimulation and to Credé and Valsalva maneuvers, (3) asking the patient to voluntarily contract and relax the external sphincter, and (4) seeing the reaction of the sphincter to filling and emptying of the bladder (Fig. 128–1) (Blaivas et al, 1977a; Blaivas, 1979b). (From Bauer SB. Urodynamic evaluation and neuromuscular dysfunction. In: Kelalis PP, Kin LR, Belman AB, editors. Clinical pediatric urology. 2nd ed, vol. 1. Philadelphia: WB Saunders; 1985. p. 283–310.) The rate of bladder filling per minute is selected by first calculating the child’s predicted or known capacity (see Chapter 127) and then dividing the result by 10 in order to fill the bladder slowly. In children with myelodysplasia, the average bladder capacity (in milliliters) fits the following formula: 24.5 × age in years + 62 (Palmer et al, 1997). It has been shown that more rapid filling rates may yield falsely low levels of detrusor compliance and may minimize an overactive detrusor (Turner-Warwick, 1975; Joseph, 1992). The bladder is filled slowly with saline warmed to 37° C to avoid this problem (Chin-Peuckert et al, 2004). When it is important to determine very mild degrees of poor compliance, even slower rates of filling are used to measure its true incidence (Kerr et al, 1994; Yeung et al, 1995; Zermann et al, 1997). Some investigators have recorded pressure at residual volume to measure natural filling pressure as the most accurate means of denoting poor compliance (Kaefer et al, 1997; Walter et al, 1998). During filling, it is helpful to try to divert the child’s attention by asking unrelated questions, reading a story aloud, or showing a movie or cartoon. If the examiner wishes to elicit detrusor overactivity, the child is asked to cough (Mayo, 1978); alternatively, a cold solution may be instilled at a rapid rate. Most urodynamic parameters do not change with a child’s position (sitting vs. supine). However, the sitting position is preferred for the detection of detrusor overactivity and incontinence (Lorenzo et al, 2007). The study is not considered complete until the child urinates and voiding pressures are measured in those children who urinate on their own. The small size of the urodynamic catheter does not seem to affect micturition pressures adversely, even in very young children. The normal voiding pressure varies from 55 to 80 cm H2O in boys and from 30 to 65 cm H2O in girls (Blaivas et al, 1977b; Blaivas, 1979a; Gierup et al, 1979). Infants tend to have higher voiding pressures than older children. Sometimes it is difficult to get the child to void; patience and time are needed in this situation. Placing a urine collection device under the buttocks of the child when supine or dripping tepid water on the genital area often stimulates the child sufficiently to begin urinating. Once the child has voided, it is important to do the following: Video-urodynamics has gained popularity since its introduction in 1970 (Bates et al, 1970). Visualization of the bladder and bladder neck during filling and of the urethra during voiding has added a dimension for more accurately integrating all aspects of lower urinary tract function and characterizing an abnormality (Glazier et al, 1997). Incompetency of the bladder neck or pelvic floor or the location of any posterior urethral obstruction can be correlated with pressure measurements and external urethral sphincter EMG activity recorded simultaneously (Zerin et al, 1990). Sometimes, aberrations noted on pressure monitoring of sphincter EMG tracings can be confirmed, enhancing these findings (Passerini-Glazel et al, 1992; Weerasinghe and Malone, 1993). Ambulatory urodynamics has become available and feasible in children with the development of microtransducers mounted on small catheters positioned in the bladder (McInerney et al, 1991; Kulseng-Hanssen and Klevmark, 1996). Often, because it is appropriate to see the effects of drug administration, studies are repeated under similar circumstances several weeks to months later. This is especially true when one is trying to treat reduced detrusor compliance or overactivity medically. To determine the maximal effect, the usual dose of most drugs is taken 2 to 3 hours before the test is performed a second time. The most commonly used drugs that affect lower urinary tract function are listed in Table 128–1, along with their appropriate dose ranges. Table 128–1 Drugs That Affect Lower Urinary Tract Function * Studies evaluating safe dosing in children have not been completed. The most common cause of neurogenic bladder dysfunction in children is abnormal development of the spinal canal and internecine spinal cord. Formation of the spinal cord and vertebral column begins at about the 18th day of gestation. Closure of the canal proceeds in a caudal direction from the cephalad end and is complete by 35 days. The exact mechanism that results in closure and what produces a dysraphic state are yet to be elucidated, but numerous factors have been implicated. The incidence was reported as 1 per 1000 births in the United States (Stein et al, 1982), but there has been a definite decrease in this rate in the past 20 years (Laurence, 1989; Lary and Edmonds, 1996; CDC, 2004; Williams et al, 2005). With the advent of prenatal screening, many affected fetuses are being identified and the pregnancies terminated before 16 weeks of gestation, thereby lowering the number of children born with this disease (Palomaki et al, 1999). The addition of folate supplements to the diet of women of childbearing age has also reduced the incidence of spina bifida worldwide (Committee on Genetics, American Academy of Pediatrics, 1999). The incidence of myelodysplasia (per 1000 live births) in the general population is 1.0, for a mother with one affected child it is 20 to 50, and for a patient with myelodysplasia it is 40 (Scarff and Fronczak, 1981; Stein et al, 1982). Therefore when the disease is already present in a family, the Medical Research Council Vitamin Study Group recommends that women of childbearing age take 4000 µg (4.0 mg) of folic acid per day beginning at least 2 months before the time they plan on becoming pregnant (Committee on Genetics, American Academy of Pediatrics, 1999). There is strong evidence that folate deficiency can lead to a myelodysplastic abnormality de novo; therefore, maternal ingestion of 400 µg of folate per day in all women of childbearing age can reduce the incidence of spina bifida by 50% (Laurence et al, 1981; MRC Vitamin Study Research Group, 1991; Czeizel and Dudas, 1992; Centers for Disease Control and Prevention, 2004). It is now mandatory that enriched grain products be fortified with folic acid (U.S. Food and Drug Administration, 1996). Since these recommendations have been followed worldwide, there has been a reduction in neural tube defects in the United States (Williams et al, 2005) and elsewhere (Botto et al, 2005). In some cases, however, despite increased folic acid intake, women may still give birth to a child with spina bifida owing to antibodies that have developed in response to the increased folate ingestion, thus negating its salutary effect (Rothenberg et al, 2004). Myelodysplasia is an all-inclusive term used to describe the various abnormal conditions of the vertebral column that affect spinal cord function. More specific labels for each abnormality include the following: a meningocele occurs when just the meninges but no neural elements extend beyond the confines of the vertebral canal; in a myelomeningocele, neural tissue, either nerve roots or portions of the spinal cord, has evaginated with the meningocele (Fig. 128–2); the term lipomyelomeningocele denotes that fatty tissue has developed with the cord structures and both are extending with the protruding sac. Myelomeningocele accounts for more than 90% of all open spinal dysraphic states (Stark, 1977). Most spinal defects occur at the level of the lumbar vertebrae, with the sacral, thoracic, and cervical areas, in decreasing order of frequency, less affected (Bauer et al, 1977) (Table 128–2). An overwhelming number of meningoceles are directed posteriorly, but on rare occasions they may protrude anteriorly, particularly in the sacral area. Usually the meningocele is made up of a flimsy covering of transparent tissue, but it may be open and leaking cerebrospinal fluid. For this reason urgent repair is necessary, with sterile precautions being followed in the interval between birth and spinal canal closure. In 85% of affected children there is an associated Arnold-Chiari malformation, in which the cerebellar tonsils have herniated down through the foramen magnum, obstructing the fourth ventricle and preventing the cerebrospinal fluid from entering the subarachnoid space surrounding the brain and spinal cord. Table 128–2 Spinal Level of Myelomeningocele Key Points: Neurogenic Bladder Dysfunction In the past several years, several centers in the United States have begun closing the defect in fetuses between 19 and 25 weeks of gestation in an attempt to improve the neurologic defect in these children (Adzick et al, 1998; Bruner et al, 1999; Holzbeierlein et al, 2000). Thus far, this procedure has not altered the incidence of abnormal findings in the lower extremities (Farmer et al, 2003) or on urodynamic assessment in the postnatal period (Koh et al, 2003), but, surprisingly, aqueduct obstruction and, consequently, hydrocephalus has not occurred at birth to the same incidence or extent (Johnson et al, 2003). It is possible that leakage of cerebrospinal fluid from the open spinal column accounts for herniation of the posterior brainstem down the foramen magnum, leading to the development of hydrocephalus. The neurologic lesion produced by the myelomeningocele can be variable, depending on what neural elements, if any, have everted with the meningocele sac. The bony vertebral level often provides little or no clue to the exact neurologic level or lesion produced. The height of the bony level may differ from the highest extent of the neurologic lesion for one to three vertebrae in either direction (Bauer et al, 1977). Furthermore, there may be differences in function from one side of the body to the other at the same neurologic level and from one neurologic level to the next, owing to asymmetry of the affected neural elements. It is important to remember that no two children have the same neurourologic defect. In addition, in 20% of affected children a vertebral bony or intraspinal abnormality occurs more cephalad than the vertebral defect and meningocele, and this can affect function in those portions of the cord. Children with thoracic or upper lumbar meningoceles often have complete reconstitution of the spine in the sacral area, and these individuals frequently have intact sacral reflex arc function involving the sacral spinal roots (in the authors’ series of patients with high-level lesions, 74% of neonates and 54% of older children were affected thusly) (Pontari et al, 1995). In fact, it is more likely that children with upper thoracic or cervical lesions will have just a meningocele and no myelomeningocele. Children with neurologic deficits at S1 or lower may also manifest a variety of findings on urodynamic testing, ranging from normal function to upper and/or lower motor neuron lesions involving either the bladder and/or the external urethral sphincter (Dator et al, 1992). Upper motor neuron lesions are characterized by an overactive detrusor, exaggerated sacral reflexes, absence of voluntary control over sphincter function, detrusor-sphincter dyssynergia (DSD), and no EMG evidence of denervation potentials in the sphincter (White and Klauber, 1976; Koff and DeRidder, 1977; Guzman et al, 1983; Capitanucci et al, 1997). The bladder tends to be thick walled (or trabeculated) with a closed bladder neck on voiding cystourethrography (VCUG) or ultrasonography. A lower motor neuron lesion is noted when an acontractile detrusor and partial or complete denervation of the external urethral sphincter with diminished or absent sacral reflexes may be seen. In these instances the bladder is usually small and smooth walled with an open bladder neck (Wilmshurst et al, 1999). The presence or absence of the bulbocavernosus reflex is an indicator (although not a foolproof one) of an upper or a lower motor neuron lesion, respectively (Schlussel et al, 1994). Approximately 10% of neonates with myelomeningocele exhibit no abnormality on urodynamic testing (Tarcan et al, 2001). Finally, the differing growth rates of the vertebral bodies and the elongating spinal cord add a dynamic factor in the developing fetus that further confounds the picture (Lais et al, 1993). Twenty-four percent of children with normal lower urinary tract function at birth develop upper motor neuron changes over time (Tarcan et al, 2001). Superimposed on all this is the Arnold-Chiari malformation, which can have a profound effect on the brainstem and pontine mesencephalic center, areas that are involved in control of lower urinary tract function. Therefore the neurologic lesion produced by this condition influences lower urinary tract function in a variety of ways and cannot be predicted just by looking at the spinal abnormality or the neurologic function of the lower extremities. Even careful assessment of the sacral area may not provide sufficient information to make a concrete inference. As a result, urodynamic evaluation in the neonatal period is now the standard of care at most pediatric centers in the United States, because it not only provides a clear picture of the function of the sacral spinal cord and lower urinary tract but also has predictive value in infants at risk for future urinary tract deterioration and progressive neurologic change (McGuire et al, 1981; Van Gool et al, 1982; Bauer, 1984b; Sidi et al, 1986). The advent of hostility scores and the reliability of urodynamic risk factors have led many clinicians to initiate prophylactic therapy in those neonates considered to be at risk for urinary tract deterioration (Perez et al, 1992). There are some, however, who do not consider urodynamic testing an important factor but rely solely on physical examination and radiologic assessment to direct their decision-making process (Hopps and Kropp, 2003), a concept not shared by many. Ideally, it would be best to perform urodynamic testing immediately after the infant is born, but the risk of spinal infection and the urgency for closure have not made this a viable option. It was accomplished in one study, however, and the results showed that 1 in 30 children (3.2%) experienced a change in neurologic status as a result of the spinal canal closure (Kroovand et al, 1990). Therefore, renal ultrasonography and measurement of residual urine are performed as early as possible after birth, either before or immediately after the spinal defect is closed. Urodynamic studies are delayed until it is safe to transport the child to the urodynamic suite and place him or her on the back or side for the test. The initial urodynamic study should be performed within the first 3 months of life. If the infant cannot empty the bladder after a spontaneous void or with a Credé maneuver, CIC is begun even before urodynamic studies are conducted. If the Credé maneuver is effective in emptying the bladder, it is performed on a regular basis instead of using CIC until the lower urinary tract can be fully investigated. The normal bladder capacity in the neonatal period is 10 to 15 mL; therefore, a residual urine volume of less than 5 mL is acceptable. Other tests that should be performed in the neonatal period include a urinalysis and culture, serum creatinine determination, and careful neurologic examination of the lower extremities. Once the spinal closure has healed sufficiently, a renal ultrasonogram is performed to reassess upper urinary tract architecture. Next, VCUG and urodynamic study are conducted. These studies meet several objectives: they provide baseline information about the radiologic appearance of the upper and lower urinary tracts as well as the condition of the sacral spinal cord and the central nervous system (CNS); they provide information that can be compared with findings on subsequent assessments, so that early signs of deterioration of urinary tract function and drainage, or of progressive neurologic denervation, can be detected; they help to identify infants at risk for urinary tract deterioration as a result of a poorly compliant or overactive detrusor or outflow obstruction from DSD, which predetermines the need to initiate prophylactic measures before any deterioration in upper urinary tract architecture and function actually takes place; and they help the physician to counsel parents about the child’s future bladder and sexual function (McGuire et al, 1981; Bauer et al, 1984; Bauer, 1984a; Sidi et al, 1986; Lais et al, 1993). If an abnormality is detected on renal ultrasonography or vesicoureteral reflux is demonstrated, a dimercaptosuccinic acid (DMSA) renal scan is recommended. Fifteen to 20 percent of neonates have an abnormal urinary tract on radiologic examination when first evaluated (Bauer, 1985); 3% have hydroureteronephrosis secondary to spinal shock, probably from the spinal canal closure (Chiaramonte et al, 1986), and 15% have abnormalities that developed in utero as a result of abnormal lower urinary tract function in the form of outlet obstruction (Bauer, 2003). Urodynamic studies in the neonatal period have shown that 63% of infants have bladder contractions. This is also true for an equivalent number of children with upper lumbar or thoracic lesions in whom the sacral spinal cord is spared, 50% of whom have detrusor overactivity (Pontari et al, 1995). Thirty-seven percent have an acontractile detrusor with compliance during filling that is either good (20%) or poor (17%) in this subgroup (Bauer et al, 1984; Bauer, 2003). EMG assessment of the external urethral sphincter demonstrates an intact sacral reflex arc with no evidence of lower motor neuron denervation in 40% of neonates; partial denervation is seen in 24%; and complete loss of sacral cord function is noted in 36% (Lais et al, 1993; Bauer, 2003). A combination of bladder contractility and external sphincter activity results in three categories of lower urinary tract dynamics: synergic (26%), dyssynergic with and without poor detrusor compliance (37%), and complete denervation (36%) (Fig. 128–3) (Bauer et al, 1984; Sidi et al, 1986; Bauer, 2003). DSD occurs when the external sphincter fails to decrease or actually increases its activity during a detrusor contraction or a sustained increase in intravesical pressure as the bladder is filled to capacity (Blaivas et al, 1986). Frequently, a poorly compliant bladder with high intravesical pressure occurs in conjunction with a dyssynergic sphincter, resulting in a bladder that empties only at high intravesical pressure (Van Gool et al, 1982; Sidi et al, 1986). Synergy is characterized by complete silencing of the sphincter during a detrusor contraction or when capacity is reached at the end of filling. Voiding pressures are usually within the normal range. Complete denervation is noted when no bioelectric potentials are detectable in the region of the external sphincter at any time during the micturition cycle or in response to sacral stimulation or a Credé maneuver. (From Bauer SB. Early evaluation and management of children with spina bifida. In: King LR, editor. Urologic surgery in neonates and young infants. Philadelphia: WB Saunders; 1988. p. 252–64.) The categorization of lower urinary tract function in this way has been extremely useful because it reveals which children are at risk for urinary tract changes, who should be treated prophylactically, who need close surveillance, and who can be monitored at shorter or longer intervals. Within the first 3 years of life, 71% of neonates with DSD had urinary tract deterioration on initial assessment or subsequent studies whereas only 17% of synergic children and 23% of completely denervated individuals developed similar changes (Fig. 128–4). Infants in the synergic group who showed deterioration did so only after they converted to a dyssynergic pattern of sphincter function. Among the infants with complete denervation, the ones who showed deterioration were those who had increased levels of urethral resistance, presumably caused by fibrosis of the skeletal muscle component of the external sphincter. Therefore, it appears that outlet obstruction is a major contributor to the development of urinary tract deterioration in these children (Fig. 128–5). Poor detrusor compliance plays an important role in this regard, especially when outlet resistance exceeds 40 cm H2O (McGuire et al, 1981; Landau et al, 1994; Tanaka et al, 1999). Detrusor compliance seems to be worse in children with high levels of outlet resistance (Ghoniem et al, 1989). Bloom and colleagues (1990) and then Park and associates (2001) noted an improvement in compliance when outlet resistance was reduced after gentle urethral dilation in these children; however, the reasons for this change are unclear, and the long-term effect of this maneuver on ultimate continence and lower urinary tract function remains uncertain. (From Bauer SB. Early evaluation and management of children with spina bifida. In: King LR, editor. Urologic surgery in neonates and young infants. Philadelphia: WB Saunders; 1988. p. 252–64.) It may be that detrusor filling pressures need to be looked at in a more critical way to determine whether they are an important factor in upper urinary tract deterioration. Landau and colleagues (1994) developed the concept of low detrusor filling pressure (<30 cm H2O) at specific volumes adjusted for age and not at maximal capacity. Applying this idea, they noted significantly improved sensitivity in predicting upper urinary tract deterioration. The ability to predict accurately which neonates are at risk for urinary tract deterioration prompted the initiation of prophylactic therapy with CIC and anticholinergic drugs (Geranoitis et al, 1988; Kasabian et al, 1992). Long-term success has been achieved in preventing reflux and hydronephrosis and in reducing the need for augmentation cystoplasty, from between 27% and 41% to between 11% and 17% (Edelstein et al, 1995; Wu et al, 1997; Kaefer et al, 1999). Others believe that aggressive observation and prompt intervention yields similar long-term results with less morbidity from catheterization (Teichman et al, 1994), but only time will delineate which avenue of treatment is most efficacious (Tanaka et al, 1999; Hopps and Kropp, 2003). Key Points: Neonatal Assessment for Neurogenic Bladder Dysfunction Because expectant treatment has revealed that infants with outlet obstruction in the form of DSD are at considerable risk for urinary tract deterioration, the idea of treating these children prophylactically has emerged as an important alternative. When CIC is begun in the neonatal period, it is easy for parents to master, even in uncircumcised boys, and for children to accept as they grow (Joseph et al, 1989). Complications such as meatitis, epididymitis, and urethral injury are rarely encountered, and urinary infections occur in fewer than 30% (Kasabian et al, 1992), although asymptomatic bacteriuria can be seen in almost 70% (Schlager et al, 2001). CIC alone or in combination with anticholinergic agents, when detrusor filling pressures exceed 40 cm H2O and voiding pressures are higher than 80 to 100 cm H2O, resulted in an incidence of urinary tract deterioration of only 8% to 10% (Geranoitis et al, 1988; Kasabian et al, 1992; Edelstein et al, 1995). This represents a significant drop in the occurrence of detrimental changes compared with children observed expectantly (McGuire et al, 1981; Bauer et al, 1984; Sidi et al, 1986; Teichman et al, 1994; Wu et al, 1997). Oxybutynin is administered in a dose of 0.2 to 0.4 mg/kg divided two or three times daily with no apparent side effects. On rare occasions when an overactive or poorly compliant bladder fails to respond to these measures, augmentation cystoplasty may be needed with cutaneous vesicostomy almost never required (Duckett, 1974; Mandell et al, 1981). Key Points: Management Principles The neurologic lesion in myelodysplasia is a dynamic disease process in which changes take place throughout childhood (Epstein, 1982; Reigel, 1983; Venes and Stevens, 1983; Oi et al, 1990), especially in early infancy (Spindel et al, 1987) and later at puberty (Begger et al, 1986), when the linear growth rate accelerates again. When a change is noted on neurologic, orthopedic, or urodynamic assessment, radiologic investigation of the CNS often reveals (1) tethering of the spinal cord (Fig. 128–6), (2) a syringomyelia, a dilation and fluid collection within the spinal cord, (3) increased intracranial pressure due to a shunt malfunction, or (4) partial herniation of the brainstem and cerebellum. Children with completely intact or only partially denervated sacral cord function are particularly vulnerable to progressive changes. Magnetic resonance imaging (MRI) is the test of choice because it reveals anatomic details of the spinal column and CNS (Just et al, 1990). However, it is not a functional study and when used alone it cannot provide exact information about a changing neurologic lesion. Sequential urodynamic testing on a yearly basis beginning in the neonatal period and continuing until the child is 5 years old provides a means of carefully monitoring these children to detect signs of change, thus offering the hope that early detection and neurosurgical intervention may help to arrest or even reverse a progressive pathologic process. Changes occurring in a group of neonates monitored in this manner involved both the sacral reflex arc and the pontine-sacral reflex interaction (Fig. 128–7) (Lais et al, 1993). Most children who experience such changes tend to do so in the first 3 years of life (Phuong et al, 2002) (Fig. 128–8). Twenty-two of 28 children in whom the neurologic picture became worse underwent a second neurosurgical procedure; 11 of them had a beneficial effect from the surgery and showed improvement in urethral sphincter function (Lais et al, 1993). Infants with only sacral level deficits have a 47% risk of deterioration (Dator et al, 1992). Even children with normal neurourologic function at birth have a 32% risk of developing a tethered spinal cord with the development of DSD with or without detrusor overactivity (Tarcan et al, 2001). (From Lais A, Kasabian NG, Dyro FM, et al. Neurosurgical implications of continuous neuro-urological surveillance of children with myelodysplasia. J Urol 1993;150:1879–83.) Figure 128–8 The propensity for a change in urethral sphincter innervation is greatest in the first year of life. (From Lais A, Kasabian NG, Dyro FM, et al. Neurosurgical implications of continuous neuro-urological surveillance of children with myelodysplasia. J Urol 1993;150:1879–83.) As a result of these developments it is recommended that all infants with myelodysplasia be monitored according to the guidelines set forth in Table 128–3. It is not enough to look at just the radiologic appearance of the urinary tract; scrutiny of the functional status of the lower urinary tract is important as well. In addition to the reasons cited previously, it may be necessary to repeat a urodynamic study if the upper urinary tract dilates secondary to impaired drainage from a poorly compliant detrusor. Table 128–3 Surveillance in Infants with Myelodysplasia* US, renal ultrasonography; UDS, urodynamic study; VCUG, voiding cystourethrography; RNC, radionuclide cystography. † Patients receiving intermittent catheterization and anticholinergic agents. ‡ If detrusor hypertonicity or reflux is already present. § Depending on degree of denervation. Vesicoureteral reflux occurs in 3% to 5% of neonates with myelodysplasia, usually in association with poor detrusor compliance, detrusor overactivity, and/or DSD (Flood et al, 1994). It is rare to find reflux in any neonate without these urodynamic findings (Bauer, 1984b; Geranoitis et al, 1988; Edelstein et al, 1995). If left untreated, the incidence of reflux in these infants at risk increases with time until 30% to 40% are afflicted by 5 years of age (Bauer, 1984a; Seki et al, 1999). Prophylactic treatment that lowers detrusor filling and voiding pressures with anticholinergic agents and empties the bladder by means of CIC significantly reduces this rising incidence of reflux (Edelstein et al, 1995). In children with reflux grades 1 to 3 (International Classification) who void spontaneously or have complete lesions with little or no outlet resistance and empty their bladder completely, management consists solely of prophylaxis with antibiotics to prevent recurrent infection. In children with high-grade reflux (grade 4 or 5), CIC is begun to ensure complete emptying. Children who cannot empty their bladder spontaneously, regardless of the grade of reflux, are treated with CIC to empty the bladder efficiently. Children with poor detrusor compliance with or without hydroureteronephrosis are also started on anticholinergic agents to lower intravesical pressure and ensure adequate upper urinary tract decompression (Flood et al, 1994). When reflux is managed in this manner there has been a dramatic response, with reflux resolving in 30% to 55% of individuals (Kass et al, 1981; Bauer, 1984b; Joseph et al, 1989; Flood et al, 1994; Agarwal et al, 1997; Hopps and Kropp, 2003). Although bacteriuria can be seen in as many as 56% of children on CIC it is not harmful except in the presence of high-grade reflux, because symptomatic urinary infection and renal scarring rarely occur with lesser grades of reflux (Kass et al, 1981; Cohen et al, 1990). Credé voiding should be avoided in children with reflux, especially those with a reactive external urethral sphincter. In this circumstance, the Credé maneuver results in a reflex response in the external sphincter that increases urethral resistance and raises the pressure needed to expel urine from the bladder (Barbalais et al, 1983) (Fig. 128–9). This has the effect of aggravating the degree of reflux and accentuating its water-hammer effect on the kidneys. Vesicostomy drainage (Duckett, 1974; Mandell et al, 1981) is rarely required today but is reserved for those infants (1) who have such severe reflux that CIC and anticholinergic medication fail to improve upper urinary tract drainage, (2) whose parents cannot adapt to the catheterization program, or (3) who are not good candidates for augmentation cystoplasty (Morrisroe et al, 2005). (From Bauer SB. Early evaluation and management of children with spina bifida. In: King LR, editor. Urologic surgery in neonates and young infants. Philadelphia: WB Saunders; 1988. p. 252–64.) The indications for antireflux surgery in this group of children are not very different from those applicable to children with normal bladder function. They include recurrent symptomatic (febrile) urinary infection while receiving adequate antibiotic therapy and appropriate catheterization techniques; persistent hydroureteronephrosis despite effective emptying of the bladder and lowering of intravesical pressure; severe reflux with an anatomic abnormality at the ureterovesical junction; reflux that persists into puberty; and the presence of reflux in any child undergoing surgery to increase bladder outlet resistance. Although some clinicians do not advocate reimplanting ureters in patients with low-grade reflux who are undergoing augmentation cystoplasty to lower intravesical pressure, this concept is not universally accepted (Nasrallah and Aliabadi, 1991). Jeffs and colleagues (1976) were the first to show that antireflux surgery can be very effective in children with neurogenic bladder dysfunction as long as it is combined with measures to ensure complete bladder emptying. Before this observation was made, the results of ureteral reimplantation were so dismal that most physicians treating these children advocated urinary diversion as a means of managing reflux (Smith, 1972; Cass, 1976). Since the advent of CIC, success rates for antireflux surgery have approached 95% (Kass et al, 1981; Woodard et al, 1981; Bauer et al, 1982; Kaplan and Firlit, 1983). Bilateral surgery for unilateral disease need not be done, because contralateral reflux does not occur postoperatively (Bauer, 1984a). The endoscopic injection of various materials has altered the management of reflux in children with myelomeningocele (Elder et al, 2004; Schlussel, 2004). A recent meta-analysis demonstrated that the success rate of endoscopic treatment is less in children with neurogenic bladder than those with normal bladder function (62% vs. 74%) (Elder et al, 2004). Studies comparing the effectiveness of open surgical to endoscopic management in this population show a significantly greater success rate for traditional open procedures (61% to 72.5% vs. 84.3% to 95.5%) (Engel, 1997; Granata, 1999). Thus, the endoscopic approach is a reasonable alternative to ureteroneocystotomy; however, the long-term outcomes are not defined. Urinary continence is becoming an increasingly important issue that demands attention at an early age. It has been shown that children who are continent have higher ratings in self-confidence and social acceptance than boys and girls who are wet; thus this issue becomes paramount as the children grow up and attend school (Moore et al, 2004). Initial attempts at achieving continence include CIC and drug therapy designed to maintain low intravesical pressures and a reasonable level of urethral resistance (Figs. 128-10 and 128-11). Although these measures can be initiated on a trial-and-error basis, it is more efficient to use exact treatment protocols based on specific urodynamic findings. As a result, urodynamic testing is performed if initial attempts with CIC and oxybutynin fail to achieve continence. Without urodynamic studies it is hard to know whether (1) a single drug is effective, (2) the dose should be increased, (3) a second drug should be added to the regimen, or (4) alternative methods of treatment (i.e., augmentation cystoplasty) should be contemplated. At this point, if poor detrusor compliance and/or overactivity have not been dealt with effectively the dose of the anticholinergic drug may be increased. If this is not successful, another anticholinergic agent may be combined with or given instead of oxybutynin (see Table 128–1). A recent study in adults with neurogenic bladder has shown the addition of a second anticholinergic agent to an existing double-dose anticholinergic agent resulted in improved urodynamic parameters and continence in 85% of patients who had failed single-agent therapy with minimal adverse effects (Amend, 2008). Glycopyrrolate is the most potent oral anticholinergic drug available today, but it may have the typical belladonna-like side effects common to all these drugs. Tolterodine, a newly approved anticholinergic drug, is equally effective as oxybutynin in reducing detrusor overactivity and improving compliance, but with fewer side effects, especially constipation (Goessl et al, 2000). Hyoscyamine produces fewer side effects still, but its potency is even less. Intravesical instillation of oxybutynin has been proven to be successful in lowering detrusor pressure and has fewer side effects compared with oral administration; serum concentrations may reach similar levels regardless of the route of intake (Greenfield and Fera, 1991; Massad et al, 1992). Inconvenience of administration, however, seems to preclude its long-term use (Kasabian et al, 1994). Trospium chloride, a recently approved quaternary ammonium compound with antimuscarinic activity that has a high binding affinity for muscarinic receptors M1, M2, and M3 but not nicotinic or cholinergic receptors holds promise because it has a more direct effect on detrusor function with fewer systemic side effects than anticholinergic agents (Rovner, 2004; Doroshyenko et al, 2005; Kim et al, 2005). Solifenacin, darifenacin, and propiverine are muscarinic receptor antagonists with high affinity for the M3 receptor. Propiverine also has calcium channel–blocking effects. These three novel agents may reduce adverse events; however, their safety and effectiveness has not yet been established in children. If conservative management with medications and CIC fail to achieve continence or prevent upper urinary tract deterioration, more aggressive intervention is warranted. In up to 80% of children, nocturnal bladder emptying has been shown to improve hydronephrosis, bladder compliance and capacity, urinary tract infections, and continence (Koff, 2005). Key Points: Continence in Neurogenic Bladder Dysfunction If urodynamic testing reveals that urethral resistance is inadequate to maintain continence because either the sphincter fails to react to increases in abdominal pressure or its resistance drops with bladder filling, α-sympathomimetic agents are added to the regimen (see Table 128–1); phenylpropanolamine is the most effective drug in this regard. Some children will ultimately require surgical intervention. A persistent poorly compliant or overactive detrusor may be treated either with enterocystoplasty (Mitchell and Piser, 1987; Sidi et al, 1987; Hernandez et al, 1994), autoaugmentation (Cartwright and Snow, 1989a, 1989b), or a combination thereof (Duel et al, 1998). Sigmoid, cecum, and small intestine, in that order, have been used to enlarge the bladder. The ileocecal segment is avoided in children with myelodysplasia because removing it might aggravate the bowel dysfunction in these children. Detubularization of the bowel is needed to minimize the intrinsic contractions of the intestinal segment and prevent it from causing intractable incontinence once it has been added to the bladder (Goldwasser et al, 1987; Hinman, 1988). Concerns have surfaced about gastric segments because they can cause hyponatremic hypochloremic metabolic alkalosis (Gosalbez et al, 1993) or the hematuria dysuria syndrome (Nguyen et al, 1996). Except for children with progressive or end-stage renal failure, gastrocystoplasty has not been recommended as a routine form of augmentation (Leonard et al, 2000; Plaire et al, 2000). With the long view of hindsight it is becoming evident that augmentation cystoplasty with any segment of the gastrointestinal tract can lead to long-term consequences in acid-base balance, vitamin B12 deficiency, fat absorption, renal function, bone metabolism, growth retardation, recurrent urinary tract infection, stone formation, and even cancer risk (Gilbert and Hensle, 2005). Consequently, there is considerable interest in developing new techniques for replacing the bladder without the need for bowel substitution (i.e., tissue-engineered autologous augments; see also Chapter 19). The well-documented development of malignancy in patients with intestinal and gastric bladder augmentation mandates careful surveillance of these patients. Most studies note a 10-year minimum lag time between the augmentation and presentation of disease. Thus, 10 years after augmentation an annual surveillance cystoscopy with site-directed biopsies at the anastomotic suture line is recommended (Soergel et al, 2004; Castellan et al, 2007; Vemulakonda et al, 2008). Symptoms and signs such as new-onset hydronephrosis, hematuria, or bladder wall thickening warrant prompt evaluation (Castellan et al, 2007; Vemulakonda et al, 2008). If bladder neck or urethral resistance is insufficient to allow adequate storage capacity, several operations are available to improve this continence mechanism. Bladder neck reconstruction can be undertaken in a variety of ways, including the Young-Dees or Leadbetter procedures (Young, 1919; Dees, 1949; Leadbetter, 1964) or the Kropp approach (Kropp and Angwafo, 1986), modified by Salle to make catheterization easier (Salle et al, 1994). A fascial sling procedure suspending the bladder neck and buttressing it against the undersurface of the pubis has been advocated enthusiastically by several clinicians (McGuire et al, 1986; Raz et al, 1988; Bauer et al, 1989). Artificially derived material, small intestine submucosa (SIS) (Colvert et al, 2002), and a rectus muscle “fascial” wrap around the bladder neck (Walker et al, 2000) have been as effective as rectus fascia in increasing bladder neck resistance and their effectiveness does not seem to dissipate over time (Elder, 1990; Castellan et al, 2005). Even though it is applicable to males it has not been as effective as in females (Nguyen et al, 2001; Castellan et al, 2005). Each of these operations, however, necessitates the use of CIC to empty the bladder postoperatively. It is most effective when combined with augmentation cystoplasty to ensure an adequate reservoir with good compliance (Castellan et al, 2005). The use of a bladder neck sling in conjunction with continent cutaneous catheterizable channel has been proposed to avoid bladder augmentation in some patients with neurogenic bladder. These children have a similar improvement in their continence level but may have a shorter interval between catheterization and require more anticholinergic agents than children with slings and augmentation (Snodgrass et al, 2009). However, this approach is controversial because of the potential risk of bladder deterioration related to increasing bladder pressures and lack of sufficient long-term follow-up. The artificial sphincter (Barrett and Furlow, 1982; Light and Scott, 1983; Light et al, 1983) also increases bladder outlet resistance, and its mechanism of action allows emptying at low urethral pressure. Any patient who can empty his or her bladder before the device is implanted should be able to do so afterward without the need for CIC. In fact, this is the only bladder neck procedure that allows for spontaneous voiding yet is compatible with CIC when needed (Herndon et al, 2003). However, children who have had bladder augmentation or an artificial sphincter placed before puberty are much more likely to require CIC to empty the bladder postpubertally (Catti, 2008). Poor detrusor compliance can develop postoperatively if the preoperative cystometrogram is not carefully scrutinized for signs of increased filling pressure (Woodside and McGuire, 1982; Bauer et al, 1986), and this may lead to progressive renal failure if not treated in a timely fashion with medication or augmentation cystoplasty (Bauer et al, 1993). Long-term results with the artificial sphincter have shown that it is a viable option in children with neurogenic bladder dysfunction (Bosco et al, 1991; Levesque et al, 1996; Kryger et al, 1999; Kryger et al, 2001; Herndon et al, 2003); however, device removal may be required in 9% to 20% (Lopez Pereira et al, 2006; Bauer, 2008; Catti, 2008). An alternative, minimally invasive option to increase bladder outlet resistance is the endoscopic injection of bulking agents in the bladder neck (Ben-Chiam et al, 1995). Various bulking agents have been used. Early reports are usually promising; however, long-term results are less favorable (Perez et al, 1996; Silveri et al, 1998; Guys et al, 2001). Long-term data on newly developed injectable agents are not available (Caione and Capozza, 2002; Misseri et al, 2005). Urinary diversion, once considered a panacea for children with myelodysplasia, has turned out to be a Pandora’s box of new clinical problems (Schwarz and Jeffs, 1975; Shapiro et al, 1975). Pyelonephritis and renal scarring, calculi, ureterointestinal obstruction, strictures of the conduit, and stomal stenosis are often encountered in children who are monitored on a long-term basis. Although antirefluxing colon conduits seem to have fewer complications, they are still not ideal. In the past 15 years very successful attempts have been made to reverse a urinary tract diversion in children who probably would not have undergone the procedure today (Hendren, 1973, 1990). Few children undergo urinary diversion now; if they do, it is in association with a continent catheterizable stoma. Continent urinary diversion with complete closure of the bladder neck (or compression via a fascial sling) has been used to provide a better quality of life for those with intractable urethral incompetence despite bladder outlet surgery or to make it easier to catheterize those individuals who cannot easily catheterize themselves via their native urethra (MacNeily et al, 2005). Several operations have been devised, but the ones that achieved the most publicity initially were the Kock pouch in adults (Kock, 1971; Skinner et al, 1987) and the Indiana reservoir in children (Rowland et al, 1987). Mitrofanoff (1980) created a continence mechanism by tunneling one end of the vermiform appendix into the bladder, as if reimplanting the ureter to prevent reflux, with the other end being brought out through the skin as a continent catheterizable stoma. This principle has been extended to the ureter. After the ureter is transected at the pelvic brim, a proximal transureteroureterostomy is performed and the cut end of the distal segment is brought to the skin as a stoma (Duckett and Snyder, 1986
Urodynamic Evaluation
TYPE
Dosage†
Minimum
Maximum
Cholinergic
Bethanechol (Urecholine)
0.7 mg/kg tid
0.8 mg/kg qid
Anticholinergic
Propantheline (Pro-Banthine)
0.5 mg/kg bid
0.5 mg/kg qid
Oxybutynin (Ditropan)
0.2 mg/kg bid
0.2 mg/kg qid
Glycopyrrolate (Robinul)
0.01 mg/kg bid
0.03 mg/kg tid
Hyoscyamine (Levsin)
0.03 mg/kg bid
0.1 mg/kg tid
Tolterodine (Detrol)
0.01 mg/kg bid
0.04 mg/kg bid
Trospium (Sanctura)*
10 mg/day
20 mg/day
Solifenacin (Vesicare)*
5 mg/day
10 mg/day
Darifenacin (Enablex)*
7.5 mg day
15 mg day
Propiverine (Detrunorm)*
0.1 mg/kg bid
0.4 mg/kg bid
α-Sympathomimetic
Phenylpropanolamine
2.5 mg/kg tid
2.5 mg/kg qid
Ephedrine
0.5 mg/kg tid
1.0 mg/kg tid
Pseudoephedrine
0.4 mg/kg bid
0.9 mg/kg tid
α-Sympatholytic
Prazosin (Minipress)
0.05 mg/kg bid
0.1 mg/kg tid
Phenoxybenzamine
0.3 mg/kg bid
0.5 mg/kg tid
β-Sympatholytic
Propranolol
0.25 mg/kg bid
0.5 mg/kg bid
Smooth Muscle Relaxant
Flavoxate (Urispas)
3.0 mg/kg bid
3.0 mg/kg tid
Dicyclomine (Bentyl)
0.1 mg/kg tid
0.3 mg/kg tid
Other
Imipramine (Tofranil)
0.7 mg/kg bid
1.2 mg/kg tid
Neurospinal Dysraphisms
Myelodysplasia
Pathogenesis
LOCATION
INCIDENCE (%)
Cervical–high thoracic
2
Low thoracic
5
Lumbar
26
Lumbosacral
47
Sacral
20
Neonatal Assessment
Findings
Management Principles
Neurologic Findings and Recommendations
SPHINCTER ACTIVITY
RECOMMENDED TESTS
FREQUENCY
Intact–synergic
Postvoid residual volume
q 4 mo
US
q 12 mo
UDS
q 12 mo
Intact–dyssynergic†
US
q 12 mo
UDS
q 12 mo
VCUG or RNC‡
q 12 mo
Partial denervation
Postvoid residual volume
q 4 mo
US
q 12 mo
UDS§
q 12 mo
VCUG or RNC‡
q 12 mo
Complete denervation
Postvoid residual volume
q 6 mo
US
q 12 mo
Management of Reflux
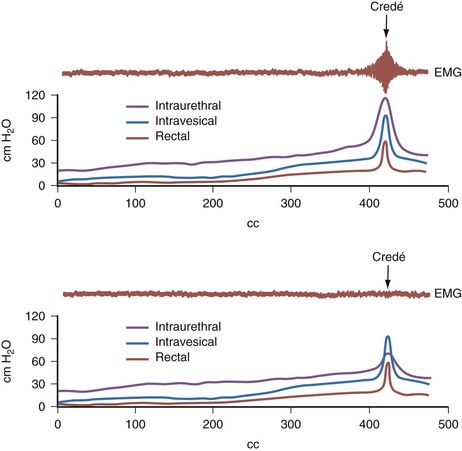
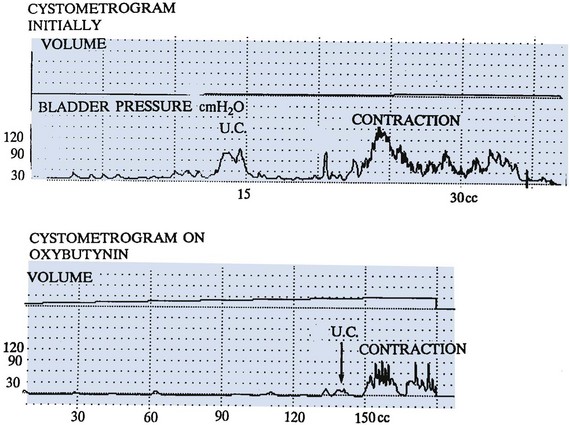
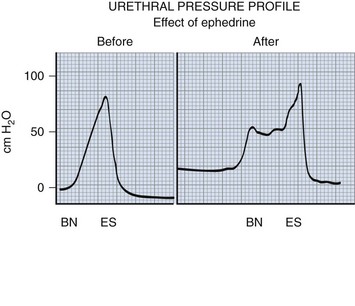
Continence and Prevention of Upper Urinary Tract Deterioration
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neuropathic Dysfunction of the Lower Urinary Tract
1. Analyze the pressure curve to determine whether the contraction is sustained until voiding is complete.
• At least 25% of the clinical problems seen in pediatric urology are the result of neurologic lesions that affect lower urinary tract function.
• Renal ultrasonography and measurement of residual urine are performed as early as possible after birth.
• Bladder pressures should be maintained below 30 cm H2O as much as possible to prevent urinary tract deterioration.
• Initial attempts at achieving continence include CIC and drug therapy designed to maintain low intravesical pressures and a reasonable level of urethral resistance.